Question: Calculate the equilibrium conversion and concentrations for the following liquid phase reaction: 2A +B 2 C + 2D Ke = 2. 93 a) Construct
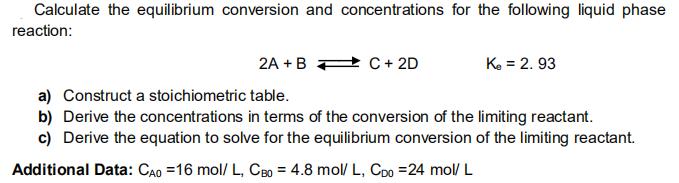
Calculate the equilibrium conversion and concentrations for the following liquid phase reaction: 2A +B 2 C + 2D Ke = 2. 93 a) Construct a stoichiometric table. b) Derive the concentrations in terms of the conversion of the limiting reactant. c) Derive the equation to solve for the equilibrium conversion of the limiting reactant. Additional Data: CAo =16 mol/ L, CB0 = 4.8 mol/ L, Cpo =24 mol/ L
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

c The equilibrium conversation of B can be determined by using equil... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


