Question: Calculate the equilibrium potentials (electrode potentials) Eo for the following electrochemical reactions as a function of the pH of the solution. It is assumed that
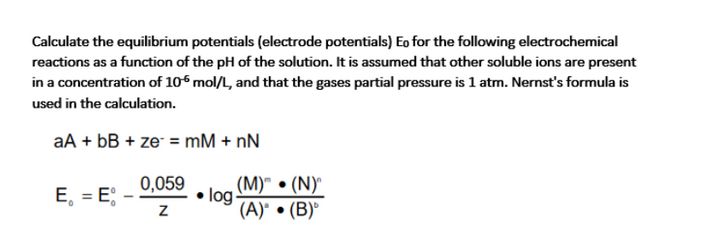
Calculate the equilibrium potentials (electrode potentials) Eo for the following electrochemical reactions as a function of the pH of the solution. It is assumed that other soluble ions are present in a concentration of 106 mol/L, and that the gases partial pressure is 1 atm. Nernst's formula is used in the calculation. aA + bB + ze = mM + nN E = E 0,059 z (M)" (N) log (A) (B)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


