Question: calculate the gold deposition potential from a hydrocyanic solution containing With: and: In standard conditions, T = 25C, and E0 = 0.60V. Au(CN)2+eAu+2CN
calculate the gold deposition potential from a hydrocyanic solution containing


With: 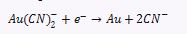
and:

In standard conditions, T = 25C, and E0 = 0.60V.
Au(CN)2+eAu+2CN
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


