Question: For this experiment, use the data shown below. Concentration of HCI (M) Initial Burette Reading Final Burette Reading Mass calorimeter cup (g) Mass calorimeter and
For this experiment, use the data shown below.
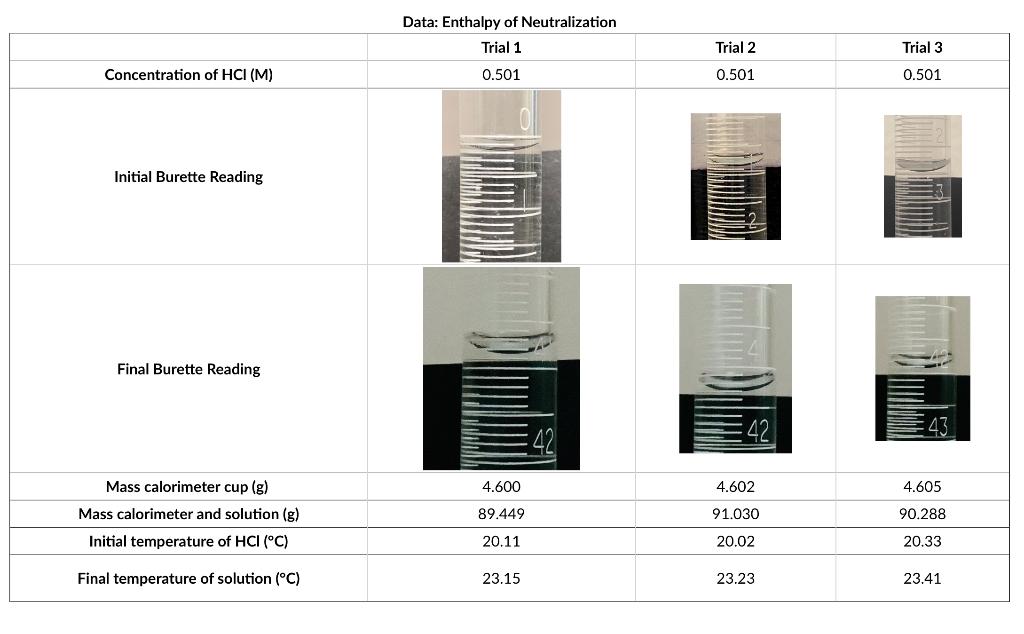



Concentration of HCI (M) Initial Burette Reading Final Burette Reading Mass calorimeter cup (g) Mass calorimeter and solution (g) Initial temperature of HCI (C) Final temperature of solution (C) Data: Enthalpy of Neutralization Trial 1 0.501 4.600 89.449 20.11 23.15 Trial 2 0.501 42 4.602 91.030 20.02 23.23 Trial 3 0.501 43 4.605 90.288 20.33 23.41
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Initial volume mL Final volume mL Mass of calorimeter cup g Mass of calorime... View full answer

Get step-by-step solutions from verified subject matter experts


