Question: Calculate the IPF for UO2, which has the CaF2 structure (Figure 3.10 ). Assume atomic radius of U4+=0.105nm,O2=0.132nm. Fions located at corners of a cube
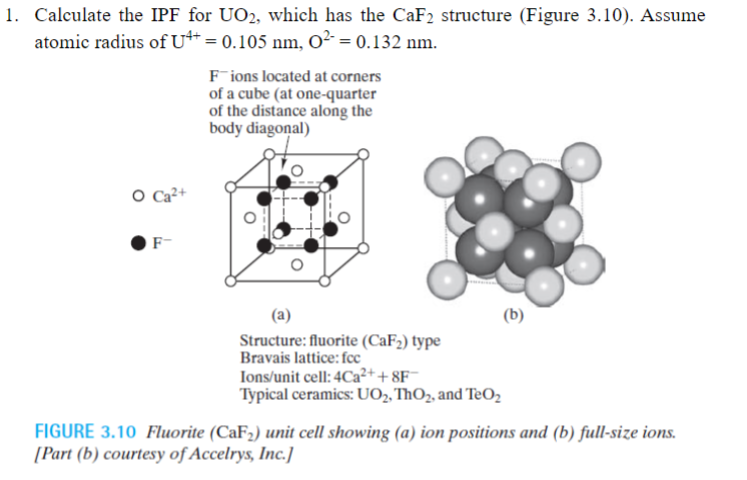
Calculate the IPF for UO2, which has the CaF2 structure (Figure 3.10 ). Assume atomic radius of U4+=0.105nm,O2=0.132nm. Fions located at corners of a cube (at one-quarter of the distance along the body diagonal) OCa2+ F (a) Structure: fluorite (CaF2) type Bravais lattice: foc Ions/unit cell: 4Ca2++8F Typical ceramics: UO2, ThO2, and TeO2 FIGURE 3.10 Fluorite (CaF2) unit cell showing (a) ion positions and (b) full-size ions. [Part (b) courtesy of Accelrys, Inc.]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


