Question: Calculate the number average and weight average relative molecu- lar mass for a sample of polystyrene made by blending eight separate fractions, each of
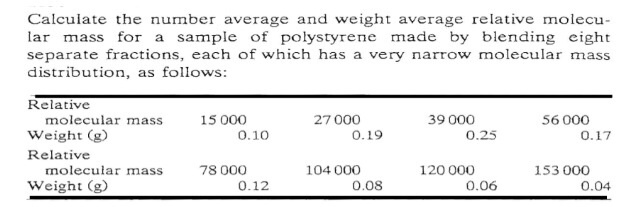
Calculate the number average and weight average relative molecu- lar mass for a sample of polystyrene made by blending eight separate fractions, each of which has a very narrow molecular mass distribution, as follows: Relative Weight (g) Relative molecular mass molecular mass Weight (g) 15 000 0.10 78 000 0.12 27 000 0.19 104 000 0.08 39 000 0.25 120 000 0.06 56 000 0.17 153 000 0.04
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
To calculate the number average Mn and weight average Mw molecular mass follow these steps Step 1 Ca... View full answer

Get step-by-step solutions from verified subject matter experts


