Question: : Calculate the specific Entropy change from the state 1 at (293K;4000kPa ) to state 2 at ( 303K;60kPa). In your calculation use the specific
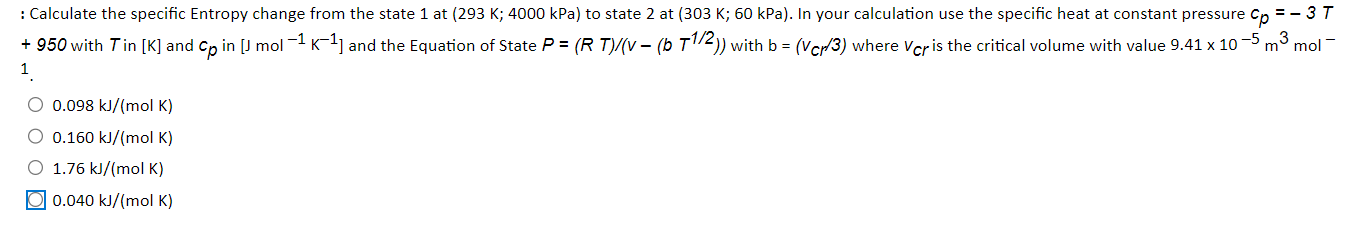
: Calculate the specific Entropy change from the state 1 at (293K;4000kPa ) to state 2 at ( 303K;60kPa). In your calculation use the specific heat at constant pressure cp=3T +950 with T in [K] and cp in [Jmol1K1] and the Equation of State P=(RT)/(v(bT1/2)) with b=(vcr/3) where vcr is the critical volume with value 9.41105m3mol 13 0.098kJ/(molK)0.160kJ/(molK)1.76kJ/(molK)0.040kJ/(molK)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


