Question: Based on a Ke value of 0.210 and the given data table, what are the equilibrium concentrations of XY, X, and Y, respectively? Express
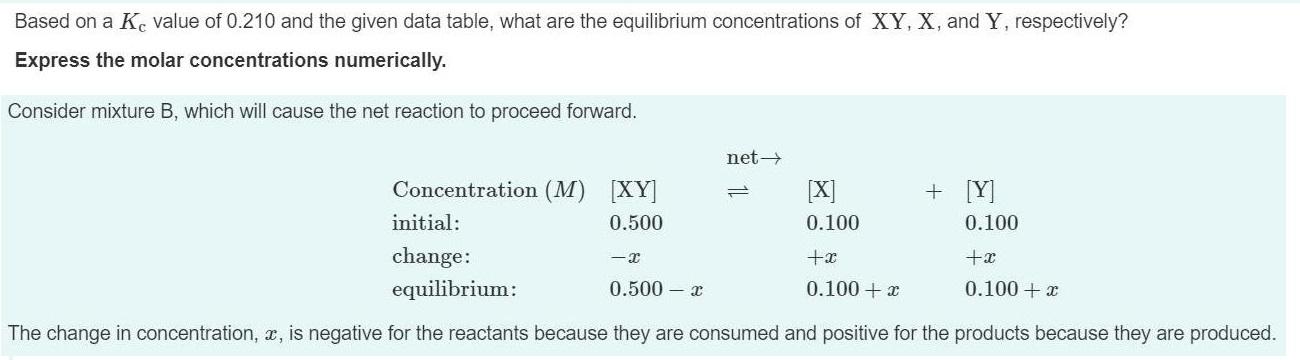
Based on a Ke value of 0.210 and the given data table, what are the equilibrium concentrations of XY, X, and Y, respectively? Express the molar concentrations numerically. Consider mixture B, which will cause the net reaction to proceed forward. net> Concentration (M) [XY] [X] + [Y] initial: 0.500 0.100 0.100 change: +x equilibrium: 0.500 x 0.100 + x 0.100 + x The change in concentration, x, is negative for the reactants because they are consumed and positive for the products because they are produced.
Step by Step Solution
3.56 Rating (156 Votes )
There are 3 Steps involved in it
Given Kc 0210 From the given reaction we can write Kc as Kc X Y XY ... View full answer

Get step-by-step solutions from verified subject matter experts


