Question: can anyone check my answer? QUESTION 1 Object (A) has a mass of 40 g and a volume of 2 ml, while object (B) has
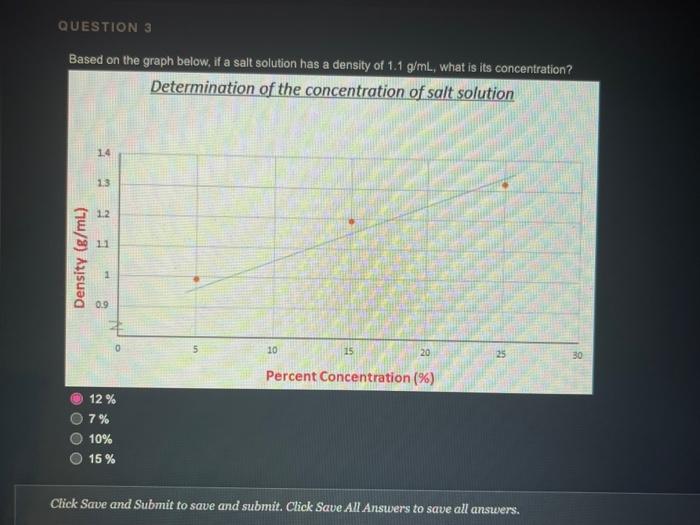
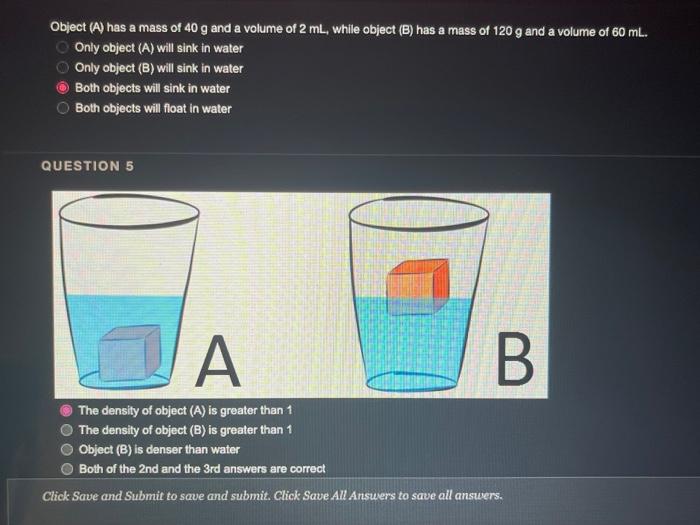
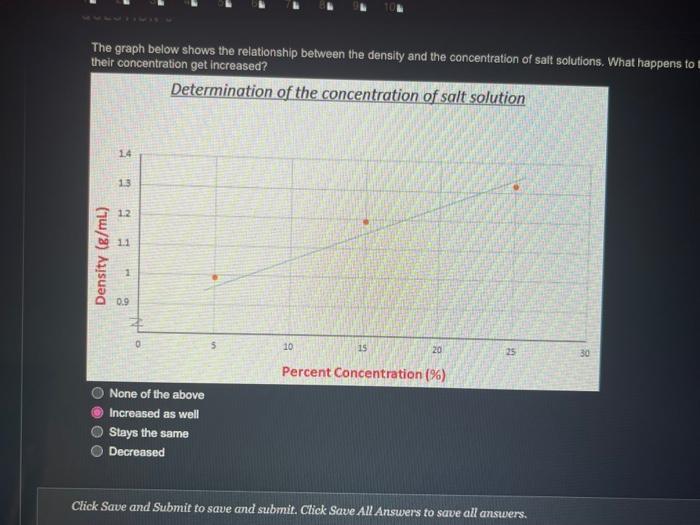
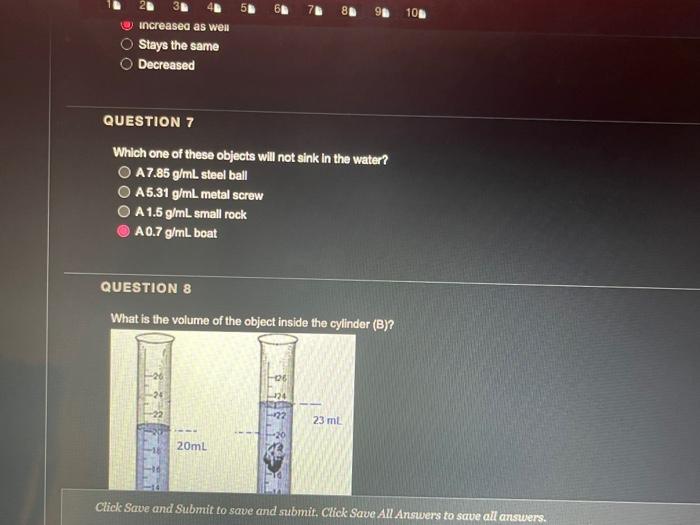
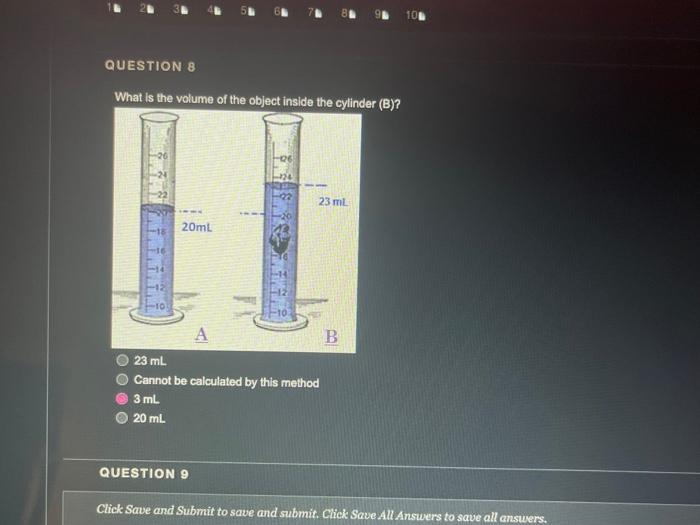
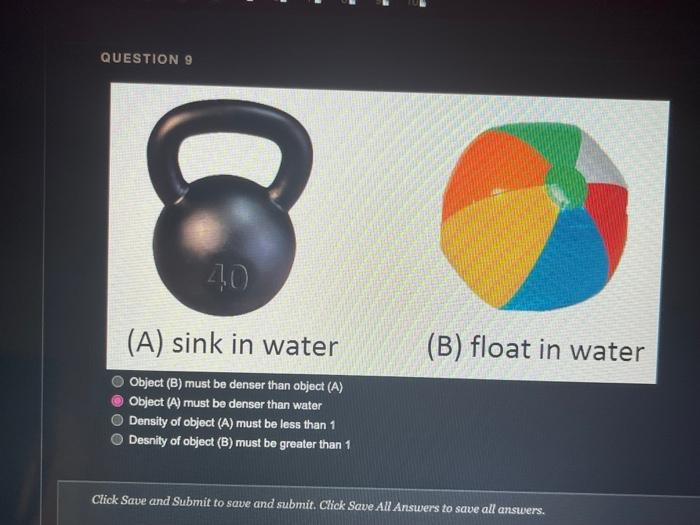
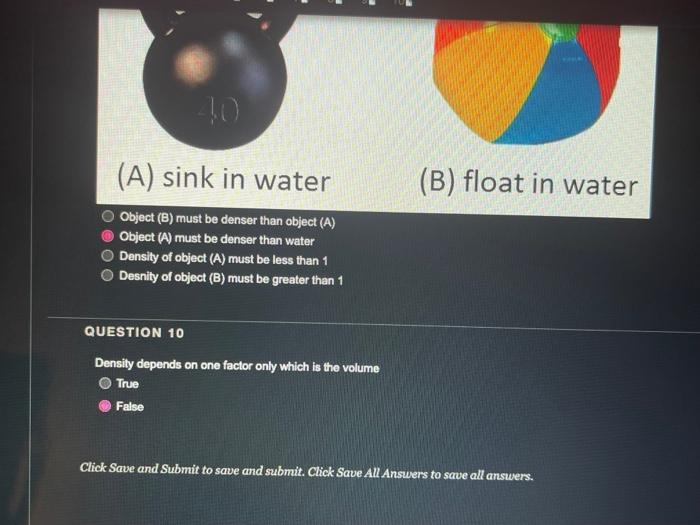
QUESTION 1 Object (A) has a mass of 40 g and a volume of 2 ml, while object (B) has a mass of 120 g and a volume of 60 ml. Object (A) is heavier and denser than object (B) Object (A) is lighter and less dense than object (8) Object (A) is heavier but less dense than object (B) Object (A) is lighter but denser than object (8) OOOO QUESTION 2 Heavy things always sink and light things always float True False QUESTION 3 Based on the graph below, if a salt solution has a density of 1.1 g/ml, what is its concentration? Determination of the concentration of salt solution 13 Click Save and Submit to save and submit. Click Save All Answers to save all answers. QUESTION 3 Based on the graph below, if a salt solution has a density of 1.1 g/ml, what is its concentration? Determination of the concentration of salt solution 14 13 12 11 Density (g/mL.) 1 0.9 0 5 10 15 20 25 30 Percent Concentration (%) 12 % 7% 10% 15 % Click Save and Submit to save and submit. Click Save All Answers to save all answers. Object (A) has a mass of 40 g and a volume of 2 ml, while object (B) has a mass of 120 g and a volume of 60 mL. Only object (A) will sink in water Only object (B) will sink in water Both objects will sink in water Both objects will float in water QUESTION 5 DA B The density of object (A) is greater than 1 The density of object (B) is greater than 1 Object (B) is denser than water Both of the 2nd and the 3rd answers are correct Click Save and Submit to save and submit. Click Save All Answers to save all answers, The graph below shows the relationship between the density and the concentration of salt solutions. What happens to their concentration get increased? Determination of the concentration of salt solution 14 13 1.2 1.1 Density (g/ml) 1 0.9 0 10 15 20 25 30 Percent Concentration (%) None of the above Increased as well Stays the same Decreased Click Save and Submit to save and submit. Click Save All Answers to save all answers. 70 100 increased as well DOO Stays the same Decreased QUESTION 7 Which one of these objects will not sink in the water? A7.85 g/mL steel ball A 5.31 g/mL metal screw A 1.5 g/mL small rock A 0.7 g/ml. boat QUESTION 8 What is the volume of the object inside the cylinder (B)? -20 -0% 320 22 23 ml 20ml Click Save and Submit to save and submit. Click Save All Answers to save all answers. 100 QUESTION 8 What is the volume of the object inside the cylinder (B)? 23 mL 20ml - TO B 23 mL Cannot be calculated by this method 3 mL 20 ml QUESTION 9 Click Save and Submit to save and submit. Click Save All Answers to save all answers. QUESTIONS 40 (A) sink in water (B) float in water Object (B) must be denser than object (A) Object (A) must be denser than water O Density of object (A) must be less than 1 Desnity of object (B) must be greater than 1 Click Save and Submit to save and submit. Click Save All Answers to save all answers. (A) sink in water (B) float in water Object (6) must be denser than object (A) Object (A) must be denser than water Density of object (A) must be less than 1 Desnity of object (B) must be greater than 1 QUESTION 10 Density depends on one factor only which is the volume True False Click Save and Submit to save and submit. Click Save All Answers to save all answers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


