Question: Can anyone explain how to get the correct answer with these graphs? A student carried out the thermodynamic experiment for borax dissolution. Using the data

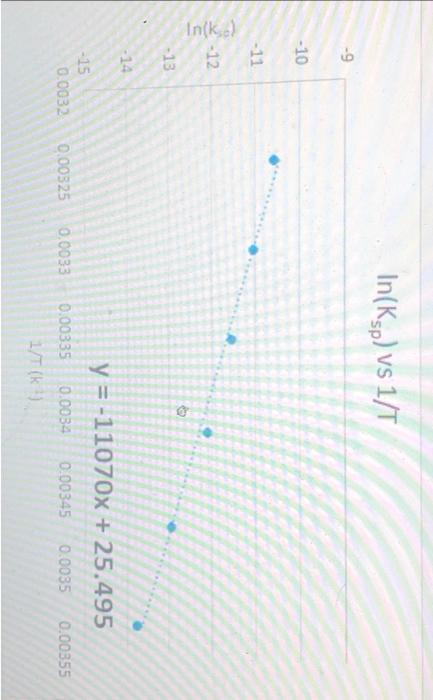
A student carried out the thermodynamic experiment for borax dissolution. Using the data obtained, student plot the graph In(Ksp) vs 1/T. The graph is given below. The equation for the best fit is given in the graph. Calculate the AG in kJmol" at 24.21C using the equation given in the graph. Record the answer to 2 decimal places. The unit of temperature is Kelvin, K. R 8.314 Jmol -9 -10 -11 In(k) -12 -13 -14 -15 0.0032 0.00325 In(Ksp) vs 1/T y = -11070x + 25.495 0.00335 0.0034 0.00345 0.0035 0.00355 1/T (k ) 0.0033
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


