Question: Can anyone please help me with these discussion problems for the extraction of caffeine from coffee grounds? Method B: Extraction of Caffeine using High Pressure/Temperature
Can anyone please help me with these discussion problems for the extraction of caffeine from coffee grounds?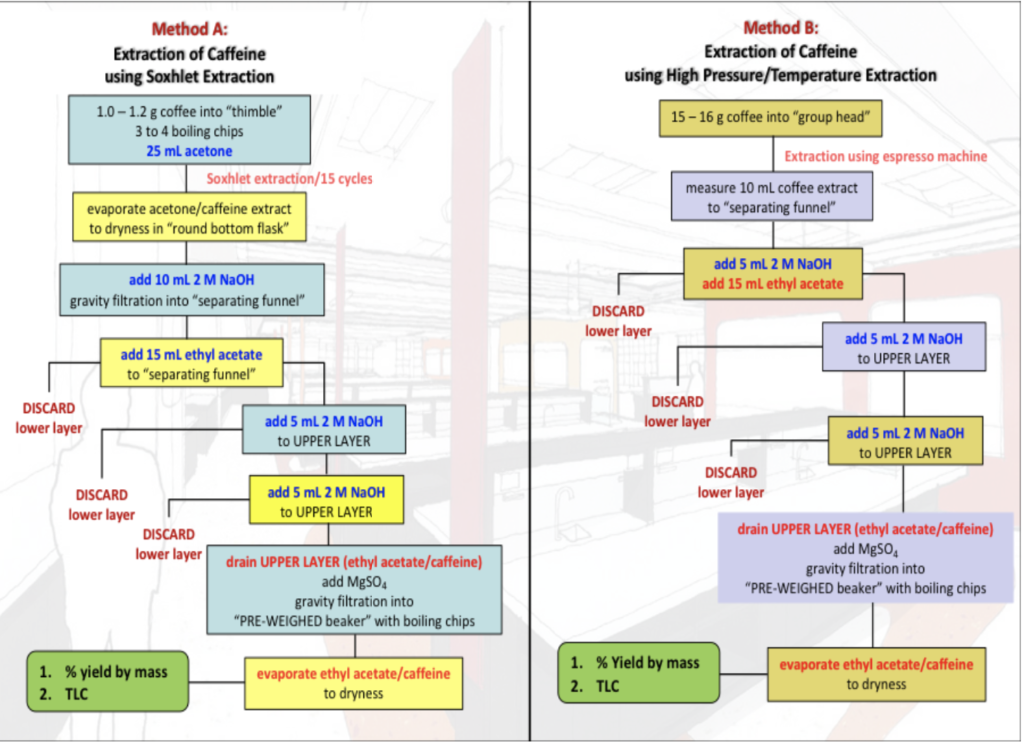

Method B: Extraction of Caffeine using High Pressure/Temperature Extraction Method A: Extraction of Caffeine using Soxhlet Extraction 1.0 - 1.2 g coffee into "thimble" 3 to 4 boiling chips 25 mL acetone Soxhlet extraction/15 cycles evaporate acetone/caffeine extract to dryness in "round bottom flask" 15- 16 g coffee into group head" Extraction using espresso machine measure 10 mL coffee extract to "separating funnel" add 10 mL 2 M NaOH gravity filtration into "separating funnel" add 5 mL 2 M NaOH add 15 mL ethyl acetate DISCARD lower layer add 5 mL 2 M NaOH to UPPER LAYER add 15 mL ethyl acetate to "separating funnel" DISCARD lower layer DISCARD lower layer add 5 mL 2 M NaOH to UPPER LAYER add 5 mL 2 M NaOH to UPPER LAYER add 5 ml 2 M NaOH to UPPER LAYER DISCARD lower layer DISCARD lower layer DISCARD lower layer drain UPPER LAYER (ethyl acetate/caffeine) add MgSO4 gravity filtration into PRE-WEIGHED beaker" with boiling chips drain UPPER LAYER (ethyl acetate/caffeine) add MgSO gravity filtration into "PRE-WEIGHED beaker" with boiling chips 1. % yield by mass 2. TLC evaporate ethyl acetate/caffeine to dryness 1. % Yield by mass 2. TLC evaporate ethyl acetate/caffeine to dryness Question 1 1. Suggest a compound that may be dissolved in the lower brown NaOH(aqueous) layer. Salt Polyphenols? tannic acid is dissolved UWhat type of intermolecular forces would be attracting this molecule into the aqueous layer? Dipole dipele Tanic acid donates a proton neutralize with NaOH Question 2 Why do you think the extract using Method B looks different i{colour, clarity) to the extract obtained by Method A (what was different about the conditions)? Question 3 (make sure to refer to your TLC results and discuss relative polarity) . Using your TLC results, what are the compound(s) extracted as caffeine and explain your answer. ii. Using your TLC results, how pure is the product extracted as caffeine and explain your answer. iii. The caffeine obtained in Method B was discoloured, however this was not observed on the TLC plate. Why not? What are the limitations of TLC? Method B: Extraction of Caffeine using High Pressure/Temperature Extraction Method A: Extraction of Caffeine using Soxhlet Extraction 1.0 - 1.2 g coffee into "thimble" 3 to 4 boiling chips 25 mL acetone Soxhlet extraction/15 cycles evaporate acetone/caffeine extract to dryness in "round bottom flask" 15- 16 g coffee into group head" Extraction using espresso machine measure 10 mL coffee extract to "separating funnel" add 10 mL 2 M NaOH gravity filtration into "separating funnel" add 5 mL 2 M NaOH add 15 mL ethyl acetate DISCARD lower layer add 5 mL 2 M NaOH to UPPER LAYER add 15 mL ethyl acetate to "separating funnel" DISCARD lower layer DISCARD lower layer add 5 mL 2 M NaOH to UPPER LAYER add 5 mL 2 M NaOH to UPPER LAYER add 5 ml 2 M NaOH to UPPER LAYER DISCARD lower layer DISCARD lower layer DISCARD lower layer drain UPPER LAYER (ethyl acetate/caffeine) add MgSO4 gravity filtration into PRE-WEIGHED beaker" with boiling chips drain UPPER LAYER (ethyl acetate/caffeine) add MgSO gravity filtration into "PRE-WEIGHED beaker" with boiling chips 1. % yield by mass 2. TLC evaporate ethyl acetate/caffeine to dryness 1. % Yield by mass 2. TLC evaporate ethyl acetate/caffeine to dryness Question 1 1. Suggest a compound that may be dissolved in the lower brown NaOH(aqueous) layer. Salt Polyphenols? tannic acid is dissolved UWhat type of intermolecular forces would be attracting this molecule into the aqueous layer? Dipole dipele Tanic acid donates a proton neutralize with NaOH Question 2 Why do you think the extract using Method B looks different i{colour, clarity) to the extract obtained by Method A (what was different about the conditions)? Question 3 (make sure to refer to your TLC results and discuss relative polarity) . Using your TLC results, what are the compound(s) extracted as caffeine and explain your answer. ii. Using your TLC results, how pure is the product extracted as caffeine and explain your answer. iii. The caffeine obtained in Method B was discoloured, however this was not observed on the TLC plate. Why not? What are the limitations of TLC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


