Question: Can explain step by step for my quiz? 2. Consider the elementary reaction 2A2B is carried out in a reactor which the catalyst decays by
Can explain step by step for my quiz?
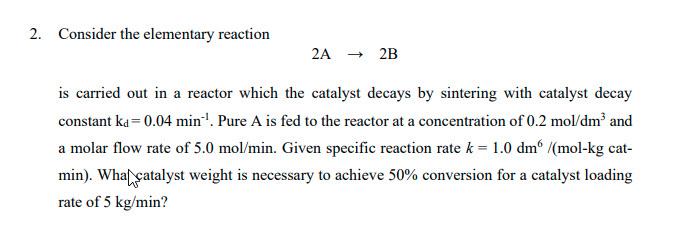
2. Consider the elementary reaction 2A2B is carried out in a reactor which the catalyst decays by sintering with catalyst decay constant kd=0.04min1. Pure A is fed to the reactor at a concentration of 0.2mol/dm3 and a molar flow rate of 5.0mol/min. Given specific reaction rate k=1.0dm6/(molkg catmin). Wha catalyst weight is necessary to achieve 50% conversion for a catalyst loading rate of 5kg/min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


