Question: can i get a quick response? Use the References to access important val: For the following reaction, 0.592 moles of hydrogen gas are mixed with
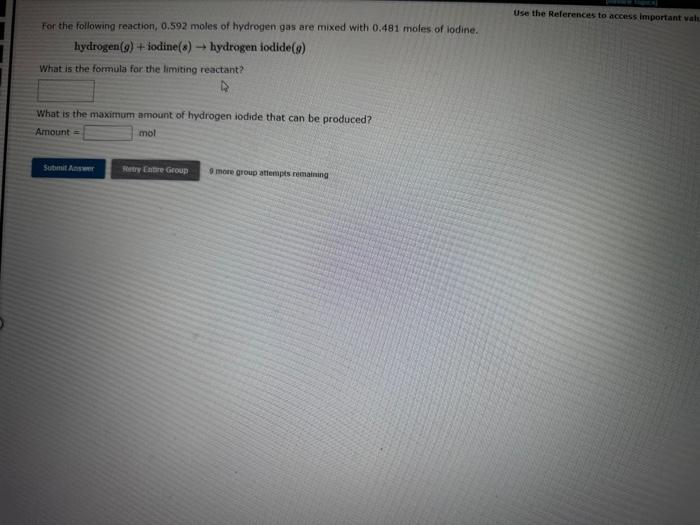
Use the References to access important val: For the following reaction, 0.592 moles of hydrogen gas are mixed with 0.481 moles of lodine. hydrogen(g)+iodine(s)hydrogeniodide(g) What is the formula for the limiting reactant? What is the maximum amount of hydrogen iodide that can be produced? Amount = mol 0 mote group atiempes remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


