Question: can i get help with this lab please (0.25pts) Use the table of water density at different temperatures to find the denisty of water at
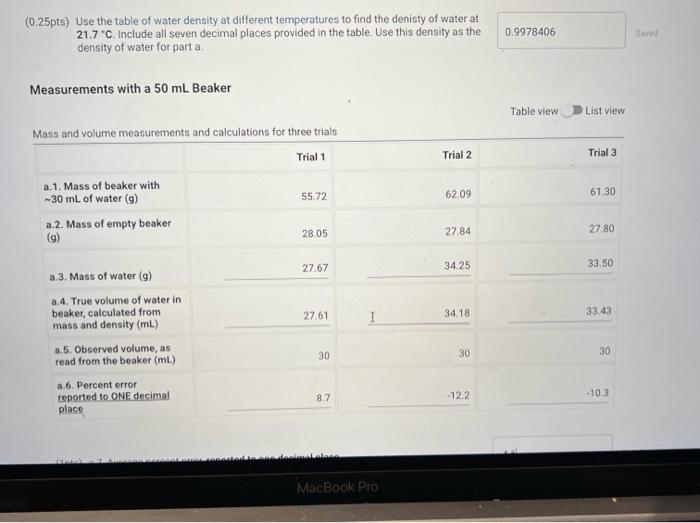
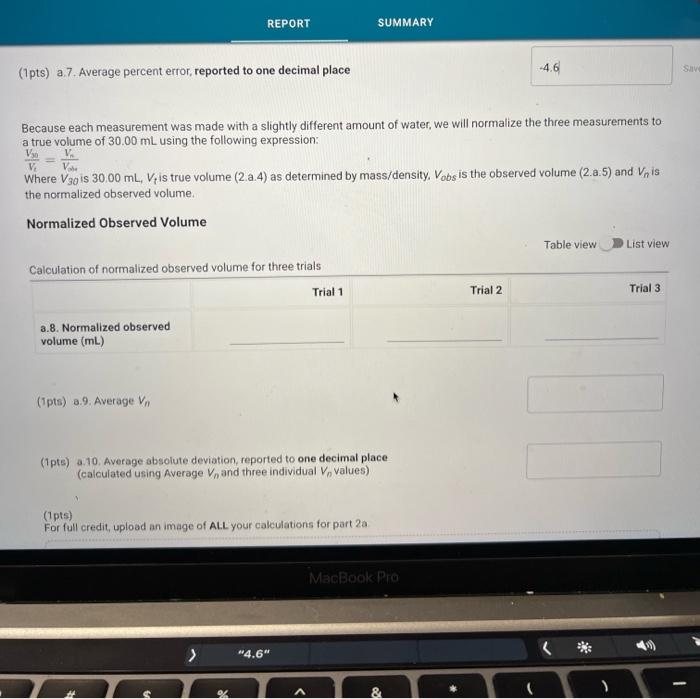
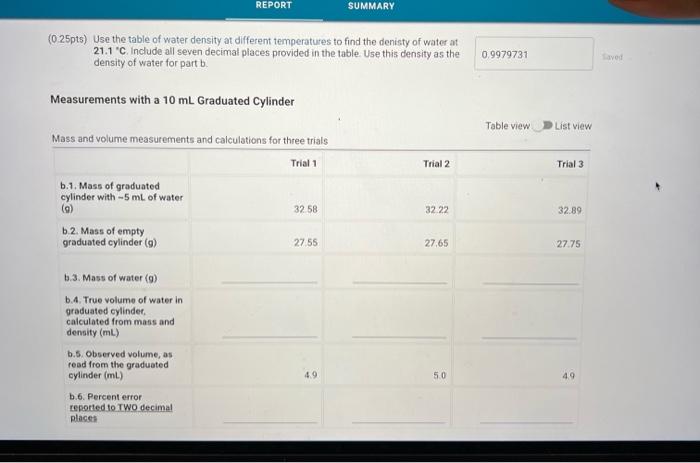
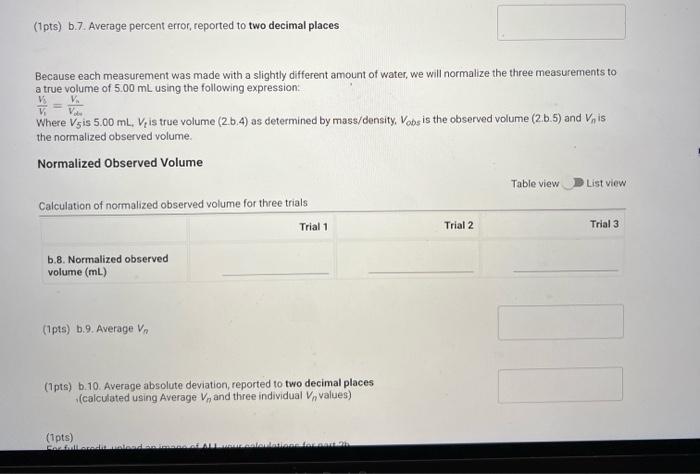
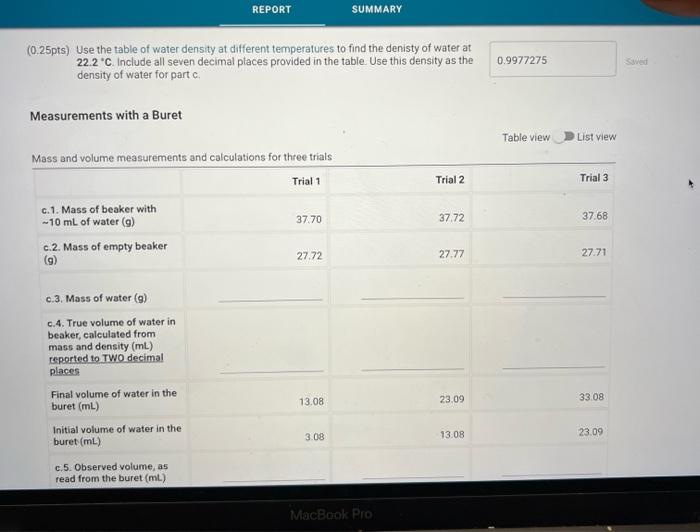
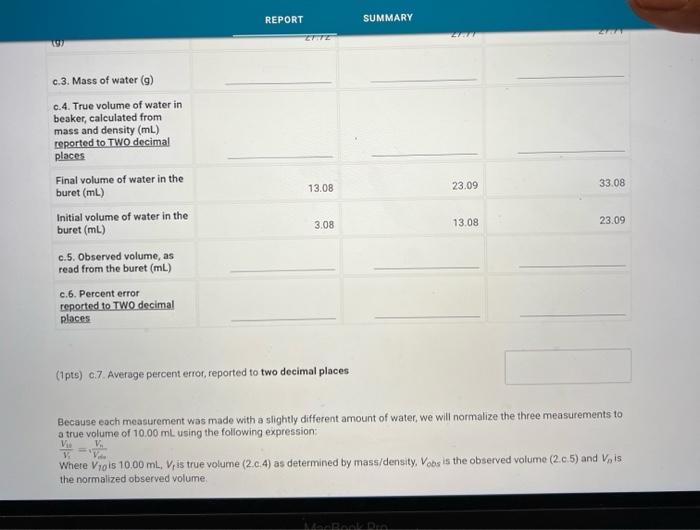
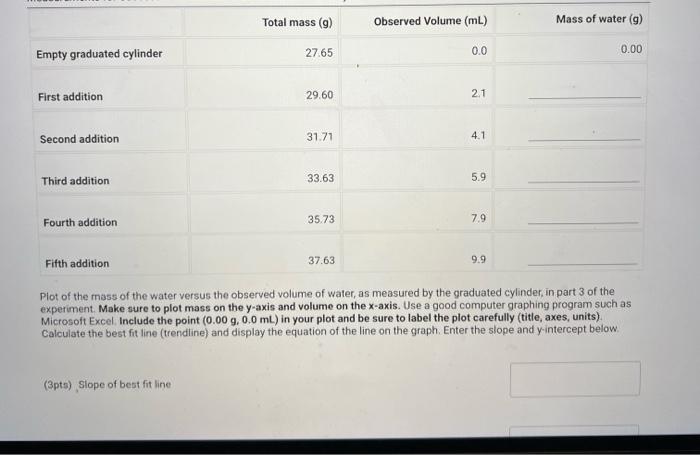
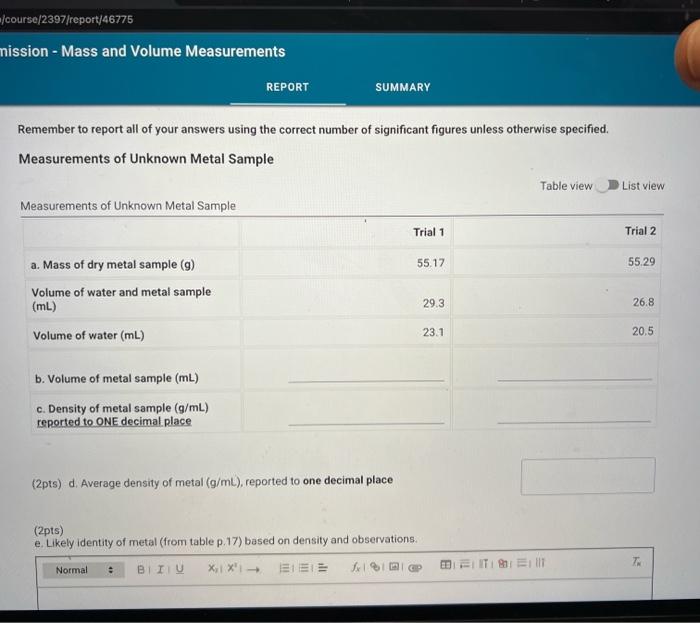
(0.25pts) Use the table of water density at different temperatures to find the denisty of water at 21.7C. Include all seven decimal places provided in the table. Use this density as the density of water for part a. Measurements with a 50mL Beaker Mass and volume measurements and calculations for three trials (1pts) a.7. Average percent error, reported to one decimal place Because each measurement was made with a slightly different amount of water, we will normalize the three measurements to a true volume of 30.00mL using the following expression: VtV30=VonVn Where V30 is 30.00mL,Vt is true volume (2.a.4) as determined by mass/density. Vobs is the observed volume (2.a.5) and Vn is the normalized observed volume. Normalized Observed Volume Table view List view polaitatina af nurmalizad nheorvarl voluma for three trials (0.25pts) Use the table of water density at different temperatures to find the denisty of water at 21.1 C. Include all seven decimal places provided in the table. Use this density as the density of water for part b. Measurements with a 10mL Graduated Cylinder Table view Dist view (1pts) b.7. Average percent error, reported to two decimal places Because each measurement was made with a slightly different amount of water, we will normalize the three measurements to a true volume of 5.00mL using the following expression: V1V0=VdiVa Where V5 is 5.00mL,Vt is true volume (2.b.4) as determined by mass/density, Vobs is the observed volume (2.b.5) and Vn is the normalized observed volume. Normalized Observed Volume Table view List view Caleidatinn of nosmalized observed volume for three trials (1pts) b.9. Average Vn (1pts) b.10. Average absolute deviation, reported to two decimal places (calculated using Average Vn and three individual Vn values) Measurements with a Buret Table view D List view (1pts) c.7. Average percent erior, reported to two decimal places Because each measurement was made with a slightly different amount of water, we will normalize the three measurements to a true volume of 10.00mL using the following expression: V1Vi=VdimVH Whete V70 is 10.00mL,Vf is true volume (2.c.4) as determined by mass/density, Vobs is the observed volume (2.c.5) and Vn is the normalized observed volume: Plot of the mass of the water versus the observed volume of water, as measured by the graduated cylinder, in part 3 of the experiment. Make sure to plot mass on the y-axis and volume on the x-axis. Use a good computer graphing program such as Microsoft Excel. Include the point (0.00g,0.0mL ) in your plot and be sure to label the plot carefully (title, axes, units) Calculate the best fit line (trendine) and display the equation of the line on the graph. Enter the slope and y-intercept below. (3pts) Slope of best fit line Remember to report all of your answers using the correct number of significant figures unless otherwise specified. Measurements of Unknown Metal Sample Table view List view (2pts) d. Average density of metal (g/mL), reported to one decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


