Question: can i please get an answer for the first picture please If 100.0kg/h of this fuel is to be burned with 30.0% excess air, what

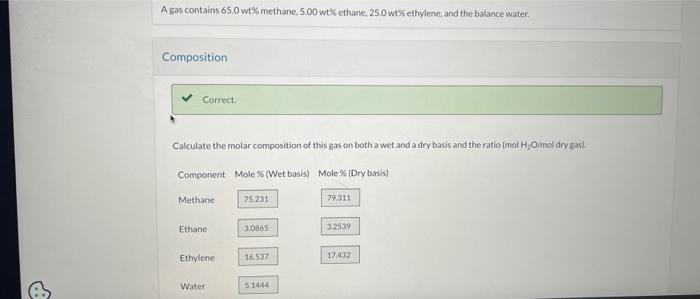

If 100.0kg/h of this fuel is to be burned with 30.0% excess air, what is the required air feed rate? kmol/h What is the required feed rate if the combustion is only 75.0% complete? kmol/h Hint Attempts: 2 A gas contains 65.0 wtK methane, 5.00wt% ethane, 25.0w%s ethylene, and the balance water. Composition Correct. Calculate the molar composition of this gas on both a wet and a dry basis and the ratio /mol2O/mol dry gasi mol H2O/mol dry gas: 0.0542
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


