Question: pls solve.. Agas contains 65.0 wt% methane, 10.00 wt% ethane, 15.0 wt% ethylene, and the balance water. Composition Your Answer Correct Answer Correct. Calculate the
 pls solve..
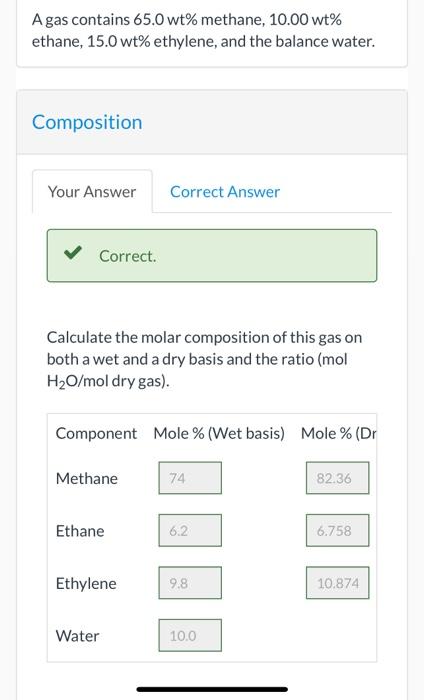
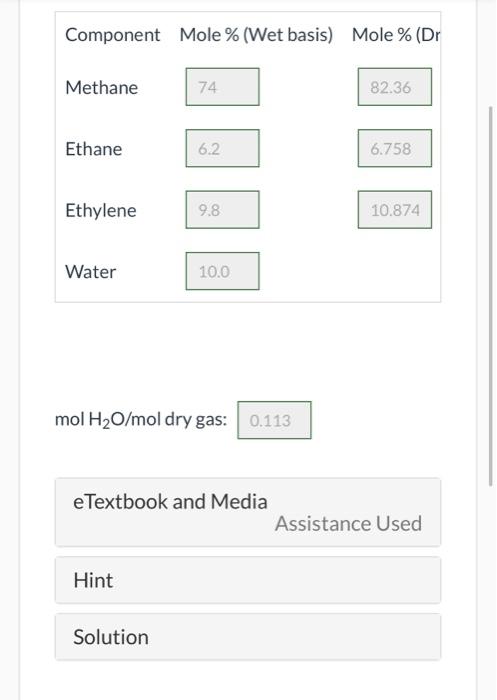
pls solve..Agas contains 65.0 wt% methane, 10.00 wt% ethane, 15.0 wt% ethylene, and the balance water. Composition Your Answer Correct Answer Correct. Calculate the molar composition of this gas on both a wet and a dry basis and the ratio (mol H2O/mol dry gas). Component Mole % (Wet basis) Mole % (Dr Methane 74 82.36 Ethane 6.2 6.758 Ethylene 9.8 10.874 Water 10.0 Component Mole % (Wet basis) Mole % (Dr Methane 74 82.36 Ethane 6.2 6.758 Ethylene 9.8 10.874 Water 10.0 mol H2O/mol dry gas: 0.113 e Textbook and Media Assistance Used Hint Solution If 100.0 kg/h of this fuel is to be burned with 30.0% excess air, what is the required air feed rate? i 70.39 kmol/h What is the required feed rate if the combustion is only 75.0% complete? i 52.79 kmol/h
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


