Question: can some please answer part b that's all the information given Imagine that 52 grams of acetylene gas (H2C2) & 160 grams of oxygen are
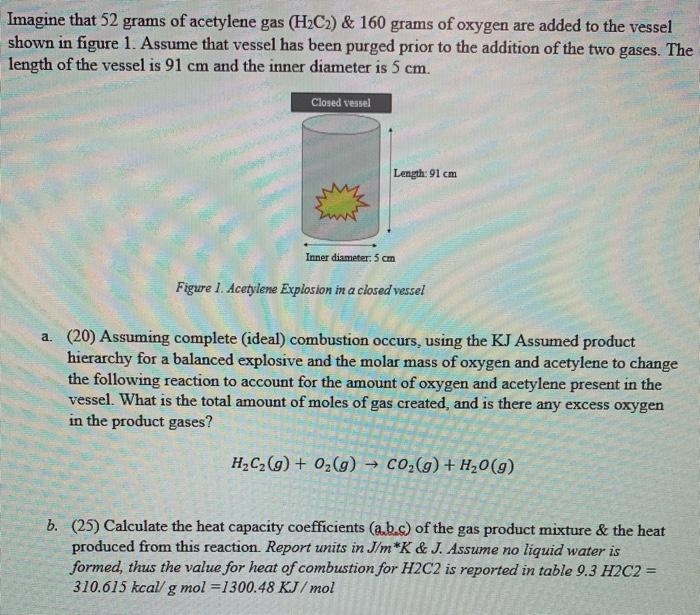
Imagine that 52 grams of acetylene gas (H2C2) & 160 grams of oxygen are added to the vessel shown in figure 1. Assume that vessel has been purged prior to the addition of the two gases. The length of the vessel is 91 cm and the inner diameter is 5 cm. Closed vessel Length: 91 cm Inner diameter: 5 cm Figure 1. Acetylene Explosion in a closed vessel a. (20) Assuming complete (ideal) combustion occurs, using the KJ Assumed product hierarchy for a balanced explosive and the molar mass of oxygen and acetylene to change the following reaction to account for the amount of oxygen and acetylene present in the vessel. What is the total amount of moles of gas created, and is there any excess oxygen in the product gases? H2C2(g) + O2(g) C02(g) + H20 (9) b. (25) Calculate the heat capacity coefficients (a.b.s) of the gas product mixture & the heat produced from this reaction. Report units in J/m*K & J. Assume no liquid water is formed, thus the value for heat of combustion for H2C2 is reported in table 9.3 H2C2 = 310.615 kcal/ g mol =1300.48 KJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


