Question: can someone answer part b,c, and d? 3. Answer ALL parts (a) Answer BOTH parts (i) and (ii). A solute with a partition coefficient of
can someone answer part b,c, and d?


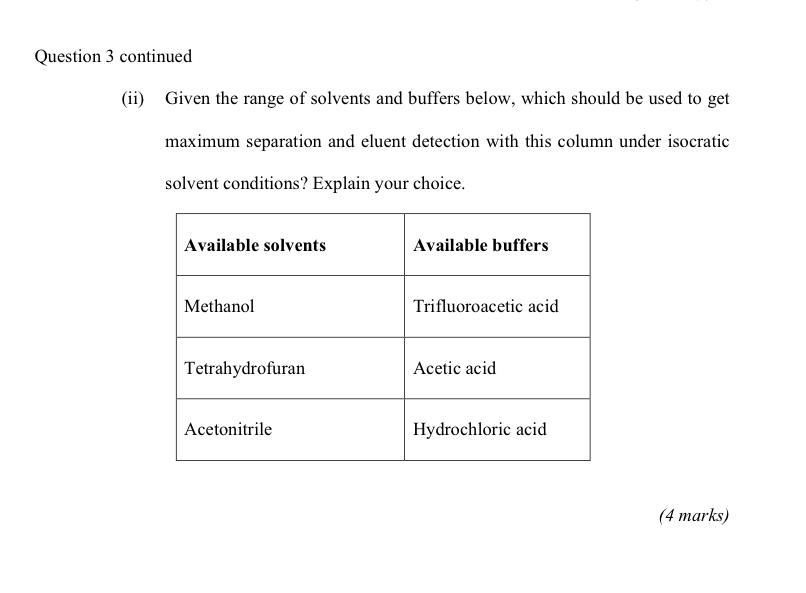
3. Answer ALL parts (a) Answer BOTH parts (i) and (ii). A solute with a partition coefficient of 3.0 is extracted from 20mL of phase 1 into phase 2. (i) What volume of phase 2 is needed to extract 99% of the solute in one extraction? (3 marks) (ii) If one extraction with 500mL volume of phase 2 is performed, what fraction of the solute remains in phase 1 ? (3 marks) (b) Answer ALL parts In a gas chromatography experiment, the relative retention for compound 1 and compound 2 is 1.068 on a column with a plate height of 0.520mm. The retention factor for compound 1,k1, is 3.29. (i) Find the retention factor for compound 2,k2. (2 marks) (ii) Given Purnell's equation for resolution, R=4N(1)(1+k2k2) what length of column will separate the compounds with a resolution R of 1.50 ? (4 marks) Question 3 continued on next page estion 3 continued (c) Answer ALL parts (i) The mobile phase strength increases as the solvent becomes less polar in reversed-phase chromatography. Explain the reason for this. (1 mark) (ii) "The mobile phase strength increases as solvent becomes more polar in normal-phase chromatography." Is this statement true? Explain the reason for your answer. (I mark) (iii) Give two differences between adsorption chromatography and partition chromatography. (4 marks) (d) Answer ALL parts A 30cm long C18 column with a plate height of 0.78mm was used for an LC-MS experiment. (i) Calculate the number of plates N for this column, and for a shorter, 150mm, column. (3 marks) Question 3 continued on next page continued (ii) Given the range of solvents and buffers below, which should be used to get maximum separation and eluent detection with this column under isocratic solvent conditions? Explain your choice. (4 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


