Question: Can someone help explaining me with this question and answering the blanks? thanks! A student desired to electroplate copper from an elemental source onto a
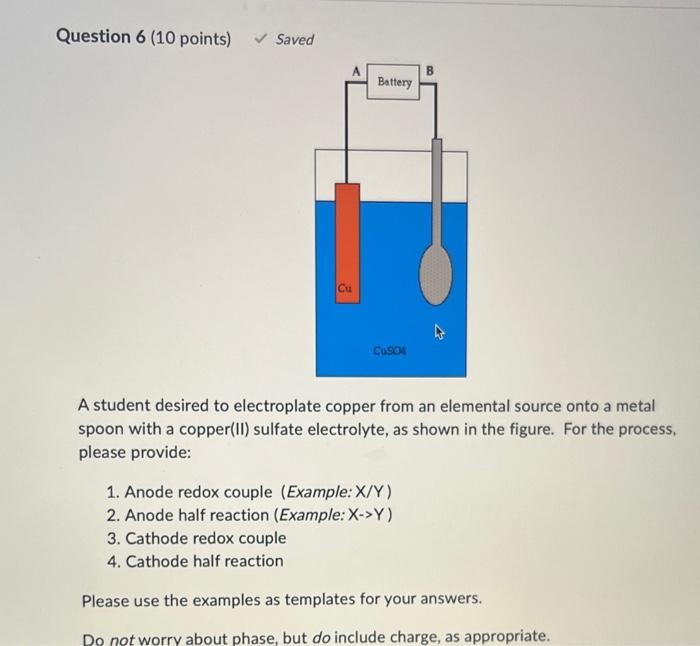

A student desired to electroplate copper from an elemental source onto a metal spoon with a copper(II) sulfate electrolyte, as shown in the figure. For the process, please provide: 1. Anode redox couple (Example: X/Y ) 2. Anode half reaction (Example: X>Y ) 3. Cathode redox couple 4. Cathode half reaction Please use the examples as templates for your answers. Do not worry about phase, but do include charge, as appropriate. Presume that the metal of the spoon is not electrochemically active under these conditions. Blank \# 1 Blank \# 2 Blank \#3 Blank \# 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


