Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.

1. b.
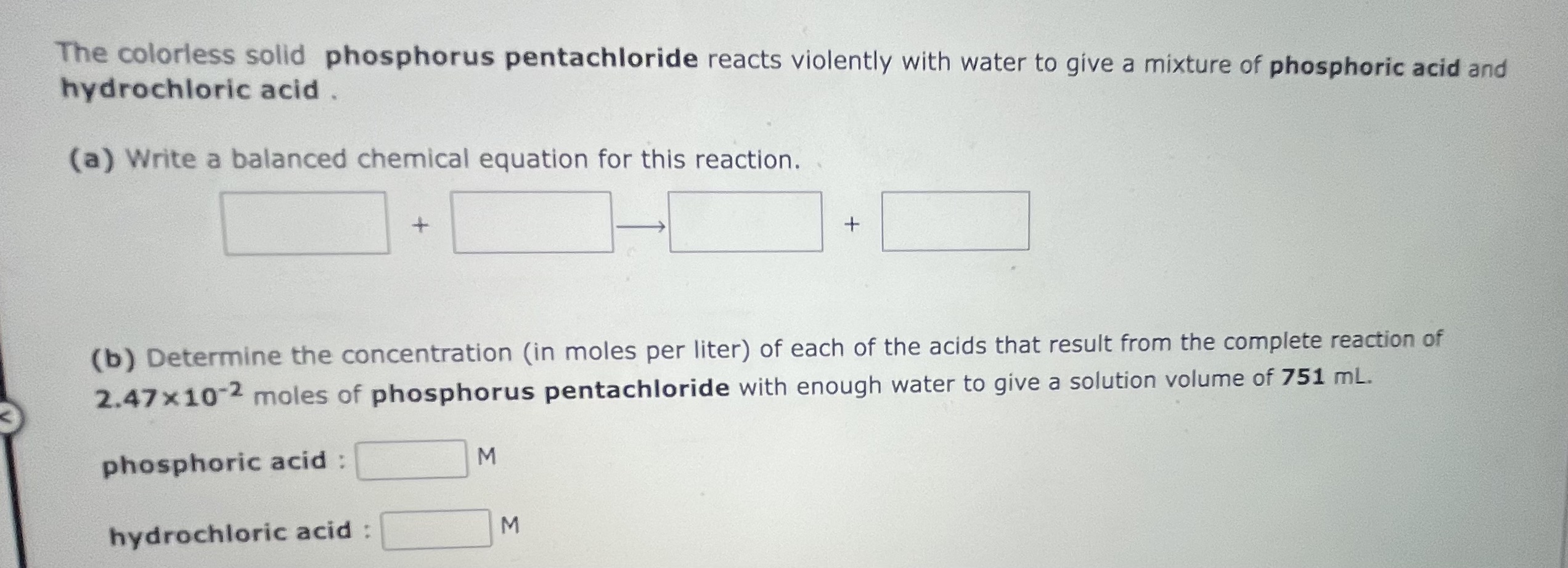
How many mL of a 0.215M aqueous solution of barium hydroxide, Ba(OH)2, must be taken to obtain 5.67 grams of the salt? The colorless solid phosphorus pentachloride reacts violently with water to give a mixture of phosphoric acid and hydrochloric acid . (a) Write a balanced chemical equation for this reaction. (b) Determine the concentration (in moles per liter) of each of the acids that result from the complete reaction of 2.47102 moles of phosphorus pentachloride with enough water to give a solution volume of 751mL. phosphoric acid : hydrochloric acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


