Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy more questions. These 3 questions are different.
1. a.

1. b.

1. c.
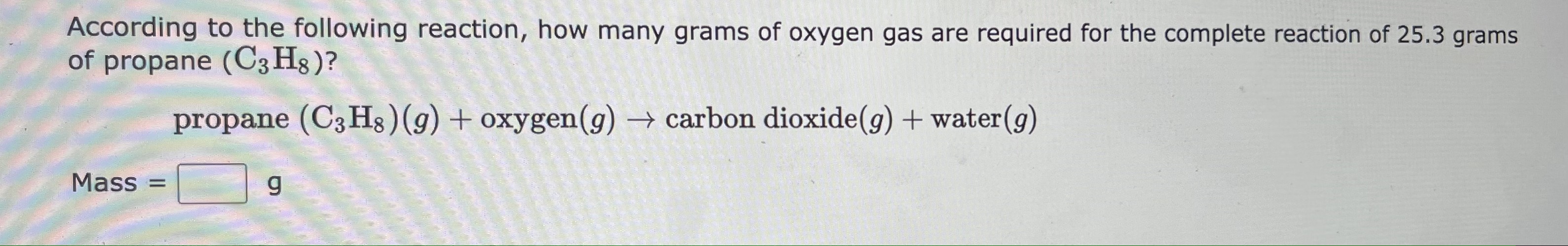
Consider the reaction: C(s)+O2(g)CO2(g) Given an initial mass of 13.11gC, an excess of O2, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of CO2 produced by the reaction. g According to the following reaction, how many grams of nitrogen monoxide will be formed upon the complete reaction of 21.2 grams of oxygen gas with excess nitrogen gas? nitrogen(g)+oxygen(g)nitrogenmonoxide(g) Mass = grams nitrogen monoxide According to the following reaction, how many grams of oxygen gas are required for the complete reaction of 25.3 grams of propane (C3H8) ? propane(C3H8)(g)+oxygen(g)carbondioxide(g)+water(g) Mass = g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


