Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.
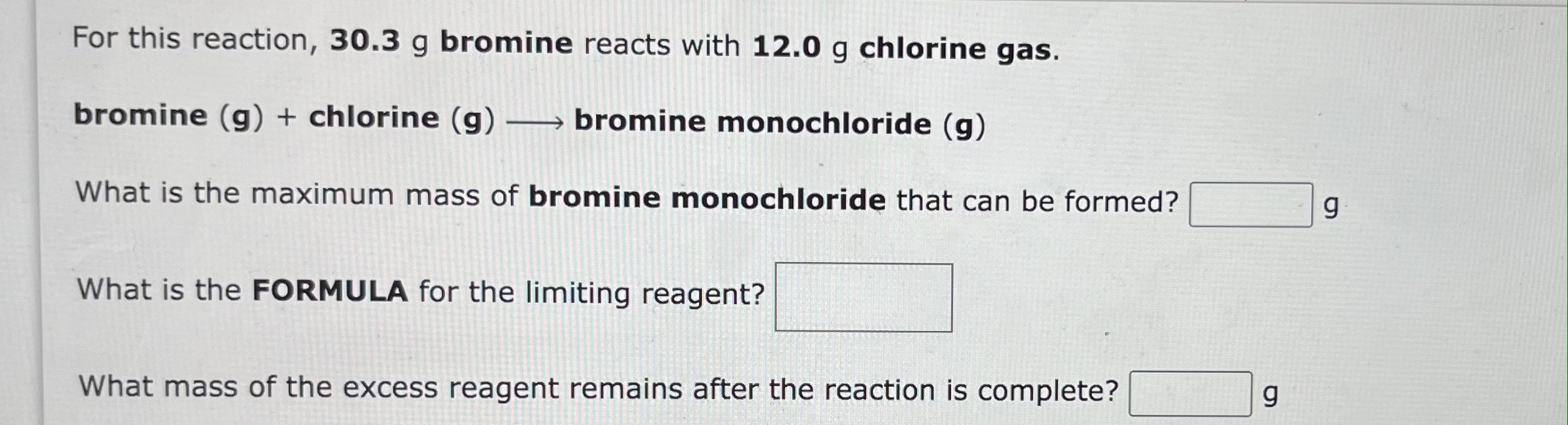
1. b.

For this reaction, 30.3g bromine reacts with 12.0g chlorine gas. bromine (g)+ chlorine (g) bromine monochloride (g) What is the maximum mass of bromine monochloride that can be formed? g What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? g For the following reaction, 61.6 grams of potassium hydroxide are allowed to react with 40.7 grams of phosphoric acid. potassiumhydroxide(aq)+phosphoricacid(aq)potassiumphosphate(aq)+water(l) What is the maximum amount of potassium phosphate that can be formed? Mass=g What is the FORMULA for the limiting reactant? What amount of the excess reactant remains after the reaction is complete? Mass =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


