Question: can someone help me with question 6 and 7 please ? Thank you 5) Using the pH table, draw the titration Curve of glycine. 6)
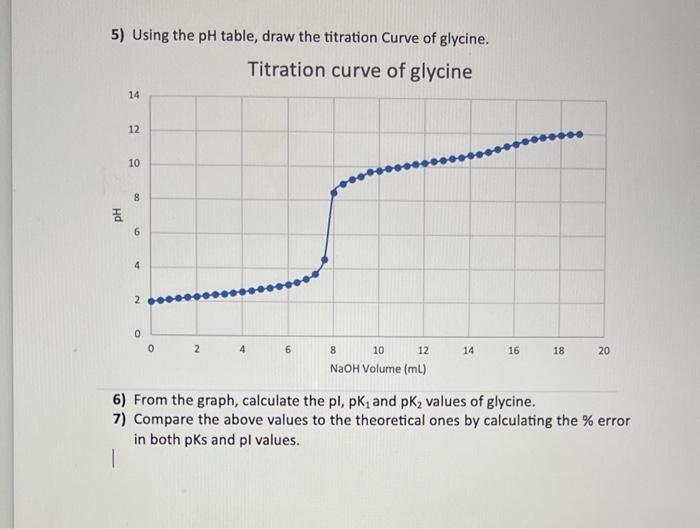
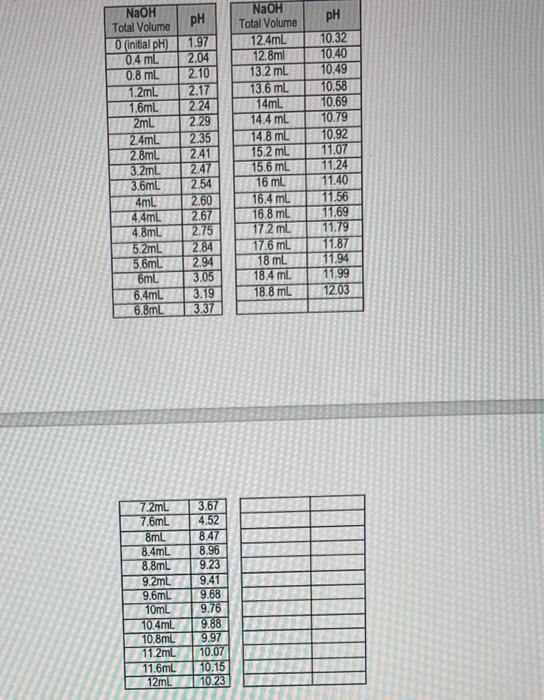
5) Using the pH table, draw the titration Curve of glycine. 6) From the graph, calculate the pl,pK1 and pK2 values of glycine. 7) Compare the above values to the theoretical ones by calculating the % error in both pKs and pl values. \begin{tabular}{|c|c|} \hline NaOH Total Volume & pH \\ \hline 0 (inital pH) & 1.97 \\ \hline 0.4mL & 2.04 \\ \hline 0.8mL & 2.10 \\ \hline 1.2mL & 2.17 \\ \hline 1.6mL & 2.24 \\ \hline 2mL & 2.29 \\ \hline 2.4mL & 2.35 \\ \hline 2.8mL & 2.41 \\ \hline 3.2mL & 2.47 \\ \hline 3.6mL & 2.54 \\ \hline 4mL & 2.60 \\ \hline 4.4mL & 2.67 \\ \hline 4.8mL & 2.75 \\ \hline 5.2mL & 2.84 \\ \hline 5.6mL & 2.94 \\ \hline 6mL & 3.05 \\ \hline 6.4mL & 3.19 \\ \hline 6.8mL & 3.37 \\ \hline \end{tabular} \begin{tabular}{|c|c|} \hline NaOH Tolal Volume & pH \\ \hline 12.4mL & 10.32 \\ \hline 12.8ml & 10.40 \\ \hline 13.2mL & 10.49 \\ \hline 13.6mL & 10.58 \\ \hline 14mL & 10.69 \\ \hline 14.4mL & 10.79 \\ \hline 14.8mL & 10.92 \\ \hline 15.2mL & 11.07 \\ \hline 15.6mL & 11.24 \\ \hline 16mL & 11.40 \\ \hline 16.4mL & 11.56 \\ \hline 16.8mL & 11.69 \\ \hline 17.2mL & 11.79 \\ \hline 17.6mL & 11.87 \\ \hline 18mL & 11.94 \\ \hline 18.4mL & 11.99 \\ \hline 18.8mL & 12.03 \\ \hline & \\ \hline \end{tabular} \begin{tabular}{|c|c|} \hline 7.2mL & 3.67 \\ \hline 7.6mL & 4.52 \\ \hline 8mL & 8.47 \\ \hline 8.4mL & 8.96 \\ \hline 8.8mL & 9.23 \\ \hline 9.2mL & 9.41 \\ \hline 9.6mL & 9.68 \\ \hline 10mL & 9.76 \\ \hline 10.4mL & 9.88 \\ \hline 10.8mL & 9.97 \\ \hline 11.2mL & 10.07 \\ \hline 11.6mL & 10.15 \\ \hline 12mL & 10.23 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


