Question: can someone help me with these problems please, if you could please do all of them i will give good rating! (a) Draw the contributing

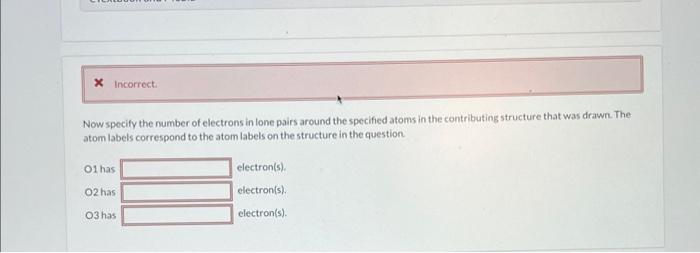
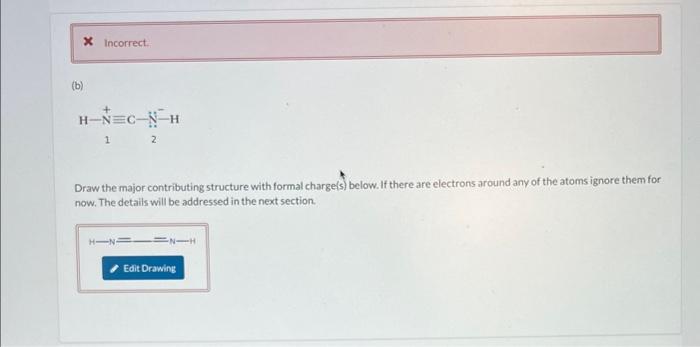

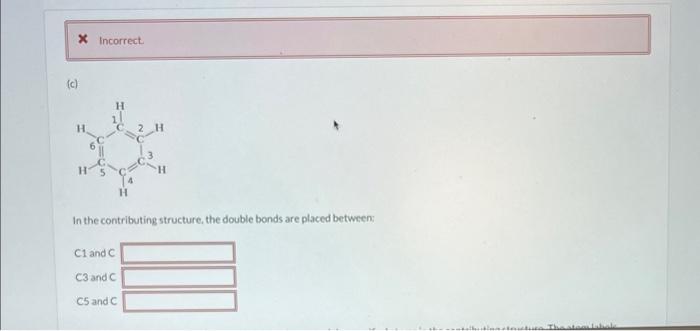

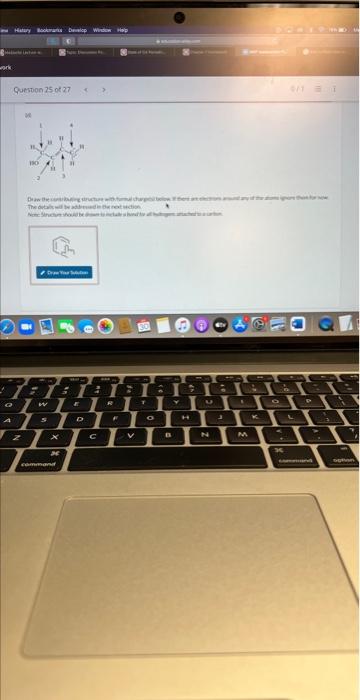

(a) Draw the contributing structure with formal charge(s) below. If there are electrons around any of the atoms ignore them for now. The details will be addressed in the next section. Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structure in the question O1hasO2hasO3haselectron(s).electron(s).electron(s). 1 2 Draw the major contributing structure with formal charge(s) below. If there are electrons around any of the atoms ignore them for now. The details will be addressed in the next section. Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structurein the question. N1 has electron(s). N2has electron(s). In the contributing structure, the double bonds are placed between: Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure. The atom labels correspond to the atom labels on the structure in the question. \begin{tabular}{l|l} C1 has & electron(s). \\ C2 has & electron(s). \\ C3 has & electron(s). \\ C4 has & electron(s). \\ C5 has & electron(s). \\ C6has & electron(s). \end{tabular} Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structure in the question. \begin{tabular}{l|l} C1 has & electron(s). \\ C2 has & electron(s). \\ C3 has & electron(s). \\ C4 has & electron(s). \end{tabular} (a) Draw the contributing structure with formal charge(s) below. If there are electrons around any of the atoms ignore them for now. The details will be addressed in the next section. Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structure in the question O1hasO2hasO3haselectron(s).electron(s).electron(s). 1 2 Draw the major contributing structure with formal charge(s) below. If there are electrons around any of the atoms ignore them for now. The details will be addressed in the next section. Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structurein the question. N1 has electron(s). N2has electron(s). In the contributing structure, the double bonds are placed between: Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure. The atom labels correspond to the atom labels on the structure in the question. \begin{tabular}{l|l} C1 has & electron(s). \\ C2 has & electron(s). \\ C3 has & electron(s). \\ C4 has & electron(s). \\ C5 has & electron(s). \\ C6has & electron(s). \end{tabular} Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structure in the question. \begin{tabular}{l|l} C1 has & electron(s). \\ C2 has & electron(s). \\ C3 has & electron(s). \\ C4 has & electron(s). \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


