Question: can someone helps me solve these problems? 2 points A sample of methane (CH4) gas having a volume of 2.80L at 25deg. C and 1.65
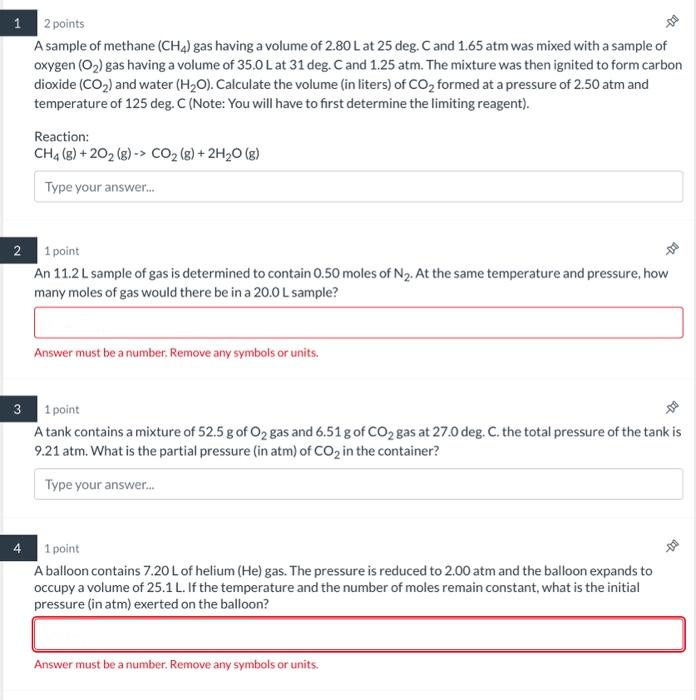
2 points A sample of methane (CH4) gas having a volume of 2.80L at 25deg. C and 1.65 atm was mixed with a sample of oxygen (O2) gas having a volume of 35.0L at 31deg.C and 1.25atm. The mixture was then ignited to form carbon dioxide (CO2) and water (H2O). Calculate the volume (in liters) of CO2 formed at a pressure of 2.50 atm and temperature of 125 deg. C (Note: You will have to first determine the limiting reagent). Reaction: CH4(g)+2O2(g)CO2(g)+2H2O(g) 1 point An 11.2 L sample of gas is determined to contain 0.50 moles of N2. At the same temperature and pressure, how many moles of gas would there be in a 20.0 L sample? 1 point A tank contains a mixture of 52.5g of O2 gas and 6.51g of CO2 gas at 27.0deg. C. the total pressure of the tank is 9.21atm. What is the partial pressure (in atm) of CO2 in the container? 1 point A balloon contains 7.20L of helium (He) gas. The pressure is reduced to 2.00atm and the balloon expands to occupy a volume of 25.1L. If the temperature and the number of moles remain constant, what is the initial pressure (in atm) exerted on the balloon? Answer must be a number. Remove any symbols or units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


