Question: Can someone please annotate this GC result for the radical chlorination of 1-chlorobutane and fill out the chart attached. Exact instructions: Tabulate yield and relative
Can someone please annotate this GC result for the radical chlorination of 1-chlorobutane and fill out the chart attached.
Exact instructions:
"Tabulate yield and relative hydrogen reactivities. Prepare a table similar to the one shown below.Relative yield comes from the GC area. Calculate theReactivity/H by dividing the yield by the number of hydrogens that give rise to that product. The relative reactivity is the normalized Reactivity/H; set the methyl group hydrogens of 1-chlorobutane (CH3CH2CH2CH2Cl)to 1.0 (corresponds to 1,4-dichlorobutane product).Statistical expectation assumes each hydrogen type has equal reactivity. Show an example calculation for each calculation type." 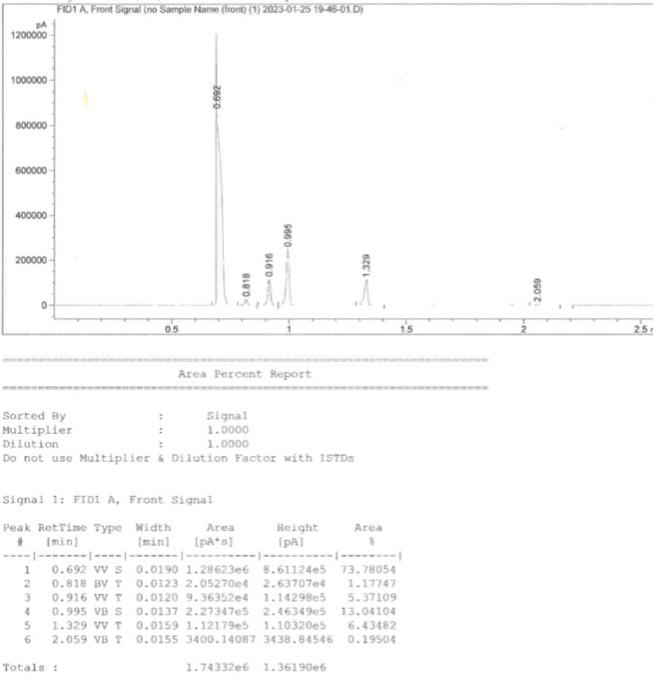
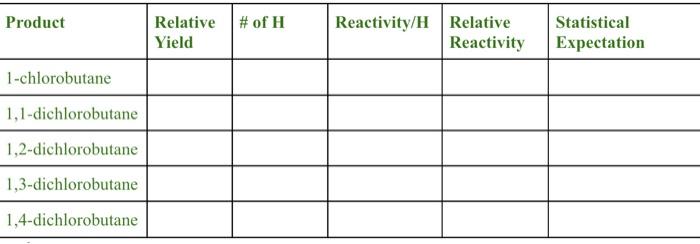


Signal 1: FID1 A, Front. Signal \begin{tabular}{l|l|l|l|l|l} \hline Product & RelativeYield & # of H & Reactivity/H & RelativeReactivity & StatisticalExpectation \\ \hline 1-chlorobutane & & & & & \\ \hline 1,1-dichlorobutane & & & & & \\ \hline 1,2-dichlorobutane & & & & & \\ \hline 1,3-dichlorobutane & & & & & \\ \hline 1,4-dichlorobutane & & & & & \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


