Question: Can someone please answer this question Given the data in the table below and Hrxn for the reaction below, SO2Cl2(g)+2H2O(l)H2SO4(l)+2HCl(g)H=184kJ/mol what is the Hf of
Can someone please answer this question
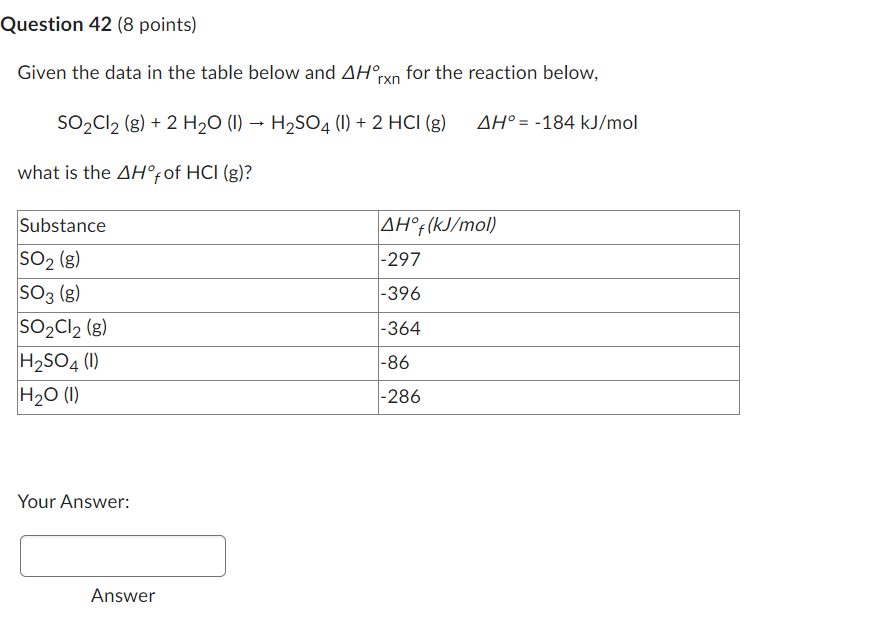

Given the data in the table below and Hrxn for the reaction below, SO2Cl2(g)+2H2O(l)H2SO4(l)+2HCl(g)H=184kJ/mol what is the Hf of HCl(g) ? Your Answer: Given the following reaction at 25 N2(g)+3H2(g)2NH3(g)K=5.7105 Calculate the equilibrium constant for the following reaction at 25 ? NH3(g)21N2(g)+23H2(g) 1.3210E3 1.3510E3 2.5510E 1.7510E6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


