Question: I NEED A LAP REPORT ANY HELP ? what information do you need ? what is the role of NaCl in the first question You
I NEED A LAP REPORT
ANY HELP ?
what information do you need ?



what is the role of NaCl in the first question
You can assume that is KCl.
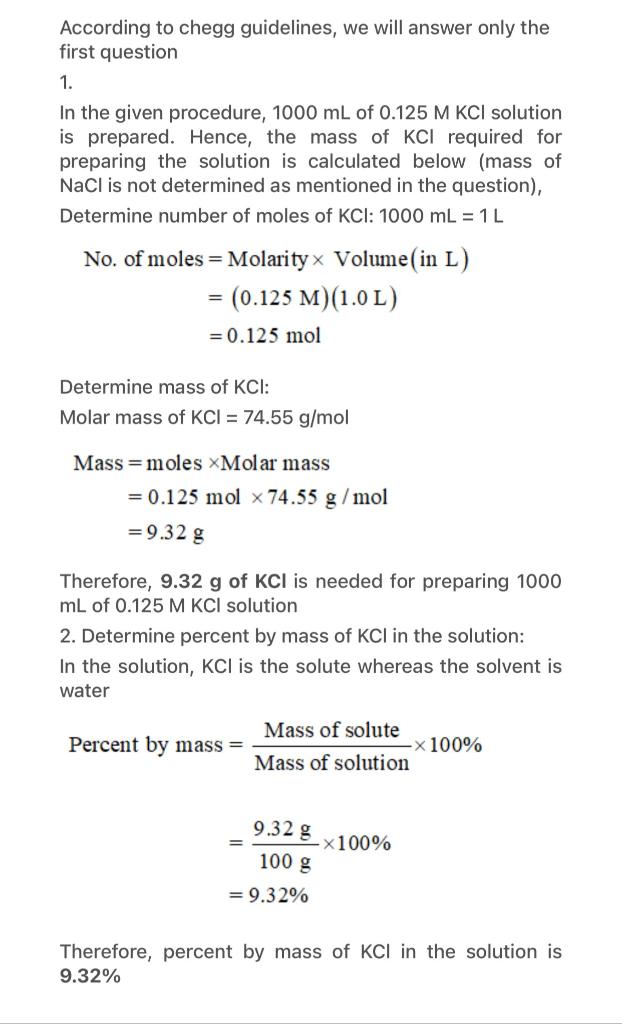
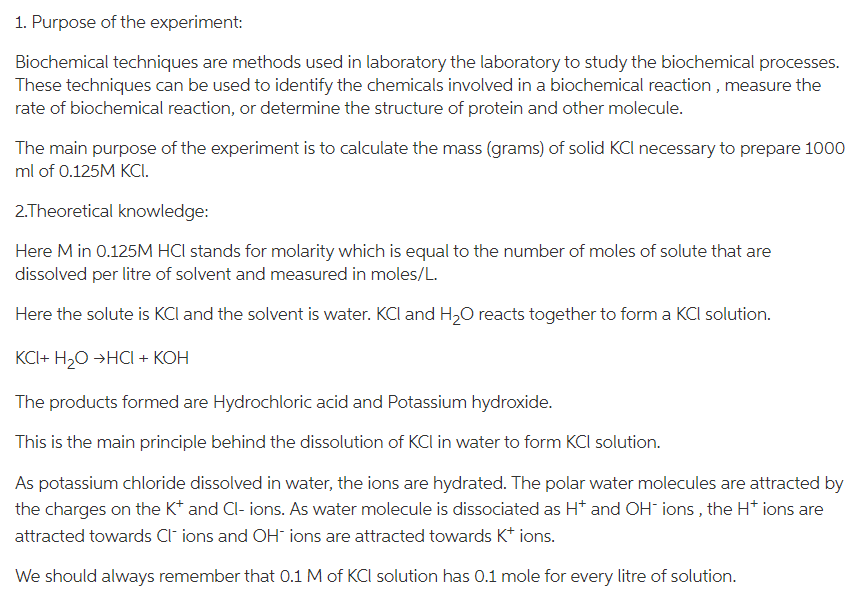
1. Laboratory Report Format The experiment reports should include the following sections: (Font: Times New Roman - Font size: 12) Title page Author name, student ID, experiment date and section, and experiment name. 1. Purpose of the Experiment (5 pt) Must answer what is the purpose of the experiment and why the experiment was carried out 2. Theory (10 pt) Provides a brief summary of the background and theory pertaining to the experiment done. (This part should not exceed one page) 3. Materials (5 pt) Materials part must include information of used consumables, devices, chemical materials. . 4. Methods (20 pt) Methods part is a step by step description of procedure in your own words. 5. Results (20 pt) State the direct outcome of the experiment or procedure. Give all the data obtained. Show all the calculations (if there is any!), writing out the equations, and defining each variable. Each calculation result should contain unit. Figures and tables can be included to present the data generated by your experiment. Tables and figures must be numbered and titled. Table titles appear at the top, figure titles at the bottom. 6. Discussion (35 pt) Restate the purpose and findings of the experiment. This section is the explanation of the results section. Include explanations of unpredicted or inconsistent results and explain. Compare results with existing knowledge. Explain why you think the results mean. Discussion should give the audience a general conclusion about the results. (If there are), answer the questions - by searching through the sources and adding your own comments. 7. References (5 pt) List the sources you used to compose the lab report. As laboratory research relies on previous work, written reports must include references. All information or interpretations given in the introduction and discussion part should be supported by references. Inadequate or inappropriate referencing should be avoided. Include references to any journal articles, books, laboratory techniques manuals, etc. used to complete your lab reports both as parenthetical references in the correct location of the text) AND in the bibliography. List references in APA format. Experiment 1: Preparing a 0.125 M KCI Solution Procedure: work as accurately as possible! 1. Start with appropriate volumetric flask. 2. Calculate the mass (grams) of solid KCl necessary to prepare 1000 mL of 0.125 M KCI. Show your work in the calculations section. 3. Use the funnel to add the KCl solute to the dry volumetric flask. 4. Fill the flask about halfway with the tap water. Be sure to rinse any solute stuck in the neck down into the flask. Swirl to dissolve the solute. 5. Add distilled water carefully to the marked line on the neck of the flask. 6. Invert the flask several times for final mixing. Calculations: 1. Calculate the mass (in grams) of solid KCl necessary to prepare 1000 mL of a 0.125 molar aqueous solution of NaCl. (Hint: Find the # of moles of NaCl, then convert to grams) 2. What is the percent by mass of KCl in your stock solution? (Hint: use mass of solution=100.0 g) Analysis 1. What is the solute in your solution? What is the solvent? 2. In your own words, describe the steps you took to make your solution. According to chegg guidelines, we will answer only the first question 1. In the given procedure, 1000 mL of 0.125 M KCl solution is prepared. Hence, the mass of KCl required for preparing the solution is calculated below (mass of NaCl is not determined as mentioned in the question), Determine number of moles of KCI: 1000 mL = 1 L No. of moles = Molarityx Volume( in L) = (0.125 M)(1.0L) = 0.125 mol Determine mass of KCI: Molar mass of KCl = 74.55 g/mol Mass=moles Molar mass = 0.125 mol x 74.55 g/mol = 9.32 g Therefore, 9.32 g of KCl is needed for preparing 1000 mL of 0.125 M KCl solution 2. Determine percent by mass of KCl in the solution: In the solution, KCI is the solute whereas the solvent is water Mass of solute Percent by mass = x 100% Mass of solution 9.32 g 100 g X100% = 9.32% Therefore, percent by mass of KCI in the solution is 9.32% 1. Purpose of the experiment: Biochemical techniques are methods used in laboratory the laboratory to study the biochemical processes. These techniques can be used to identify the chemicals involved in a biochemical reaction, measure the rate of biochemical reaction, or determine the structure of protein and other molecule. The main purpose of the experiment is to calculate the mass (grams) of solid KCl necessary to prepare 1000 ml of 0.125M KCI. 2.Theoretical knowledge: Here Min 0.125M HCl stands for molarity which is equal to the number of moles of solute that are dissolved per litre of solvent and measured in moles/L. Here the solute is KCl and the solvent is water. KCl and H2O reacts together to form a KCl solution. KCI+ H2O HCI + KOH The products formed are Hydrochloric acid and Potassium hydroxide. This is the main principle behind the dissolution of KCl in water to form KCl solution. As potassium chloride dissolved in water, the ions are hydrated. The polar water molecules are attracted by the charges on the Kt and Cl- ions. As water molecule is dissociated as Ht and OH-ions, the H+ ions are attracted towards Cl-ions and OH ions are attracted towards K+ ions. We should always remember that 0.1 M of KCl solution has 0.1 mole for every litre of solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


