Question: Can someone please help me solve this for my homework? Based on the phase diagram of CO2 shown below describe the state changes. 729 atm
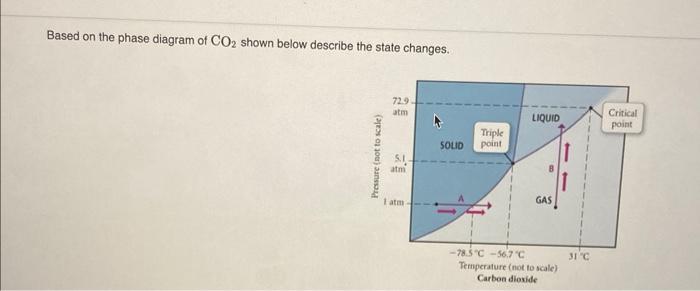

Based on the phase diagram of CO2 shown below describe the state changes. 729 atm LIQUID Critical point Triple point SOLID Pressure (not to scale) am 8 1 at GAS 31 -78.5C -56.7" Temperature (not to scale) Carbon dioxide Match the equations in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Reset Help CO) triple point at 216.5 K-C0() just above 216.5 The phase changes that occur when the temperature of Co, in increased from 100 K to 350 K at a constant pressure of 1 atm represents The phase changes that occur when the temperature of CO, is increased from 190 K 10 350 Kata constant pressure of 5.1 atm CO.() - CO (l) at somewhat above 216 K - CO.() at around 250 K 00,() - CO.(1) at somewhat above 220K -CO, above the critical point where there is no distinction between liquid and gas. This change occurs at about 305 K The phase changes that occur when the formperature of Co, is increased from 190 K 10350 Kata constant pressure of 10 atm The phase changes that occur when the temperature of Co, is increased from 190 K 1350 Kata constant pressure of 100 atm CO.() CO.() at 1947K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


