Based on the phase diagram of CO 2 shown in Figure 12.39(b), describe the state changes that
Question:
Based on the phase diagram of CO2 shown in Figure 12.39(b), describe the state changes that occur when the temperature of CO2 is increased from 190 K to 350 K at a constant pressure of
(a) 1 atm,
(b) 5.1 atm,
(c) 10 atm, and
(d) 100 atm.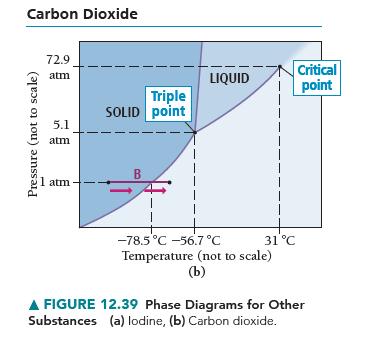
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





