Question: Can someone please help me with Part 1 and Part 2 Which of the following compounds does not have a double bond? C2H4 S2 H2CO
Can someone please help me with Part 1 and Part 2 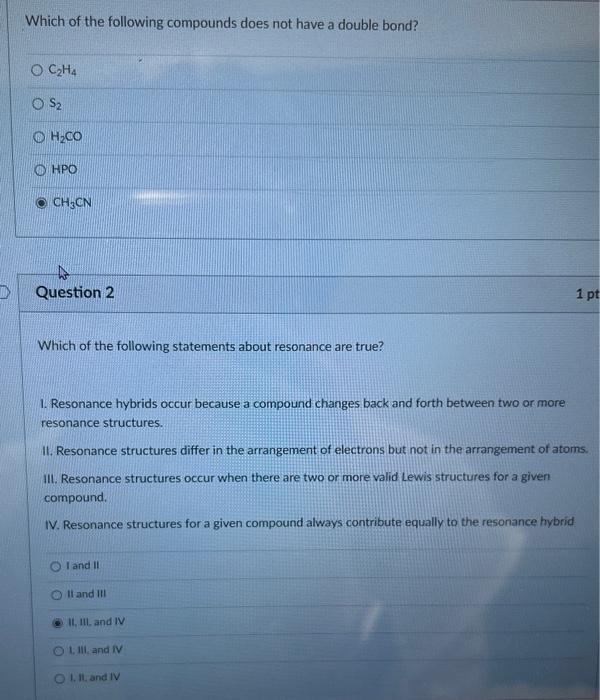

Which of the following compounds does not have a double bond? C2H4 S2 H2CO HPO HP3CN Question 2 Which of the following statements about resonance are true? 1. Resonance hybrids occur because a compound changes back and forth between two or more resonance structures. II. Resonance structures differ in the arrangement of electrons but not in the arrangement of atoms III. Resonance structures occur when there are two or more valid Lewis structures for a given compound. IV. Resonance structures for a given compound always contribute equally to the resonance hybrid I and II II and III 11. III. and IV 1. 11i, and iv 1. I1. and IV
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


