Question: Can someone please help me with this? You need to make 200.0mL of a buffer composed of NH3 and a salt of its conjugate acid.
Can someone please help me with this?
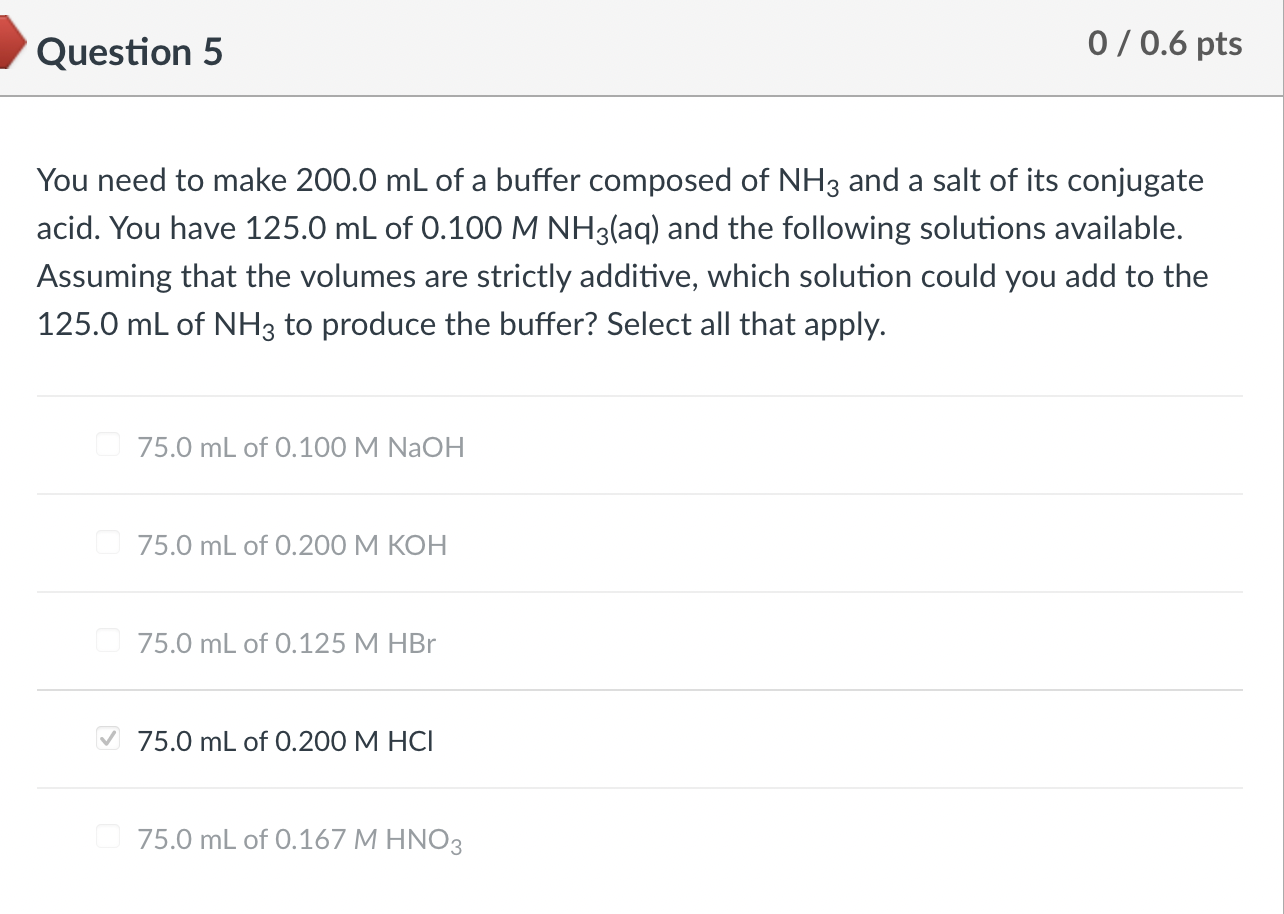
You need to make 200.0mL of a buffer composed of NH3 and a salt of its conjugate acid. You have 125.0mL of 0.100MNH3(aq) and the following solutions available. Assuming that the volumes are strictly additive, which solution could you add to the 125.0mL of NH3 to produce the buffer? Select all that apply. 75.0mL of 0.100MNaOH 75.0mL of 0.200MKOH 75.0mL of 0.125MHBr 75.0mL of 0.200MHCl 75.0mL of 0.167MHNO3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


