Question: Can someone please show a complete and detailed solution for this one? The answers are 2.3 hours and 465 cu. ft. Thanks For the reaction
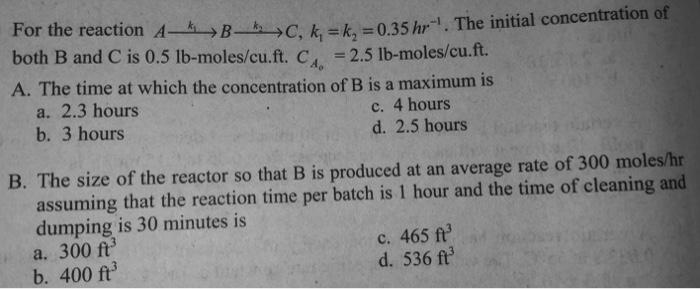
For the reaction Ak1Bk2C,k1=k2=0.35hr1. The initial concentration of both B and C is 0.5lbmoles/ cu.ft. CA0=2.5lb-moles/cu.ft. A. The time at which the concentration of B is a maximum is a. 2.3 hours c. 4 hours b. 3 hours d. 2.5 hours B. The size of the reactor so that B is produced at an average rate of 300moles/hr assuming that the reaction time per batch is 1 hour and the time of cleaning and dumping is 30 minutes is c. 465ft3 a. 300ft3 b. 400ft3 d. 536ft3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


