Question: Can someone solve these no explanation needed as long as they r right. (just dont use ChatGPT or ai in general it isn't smart lol)
Can someone solve these no explanation needed as long as they r right. (just dont use ChatGPT or ai in general it isn't smart lol)

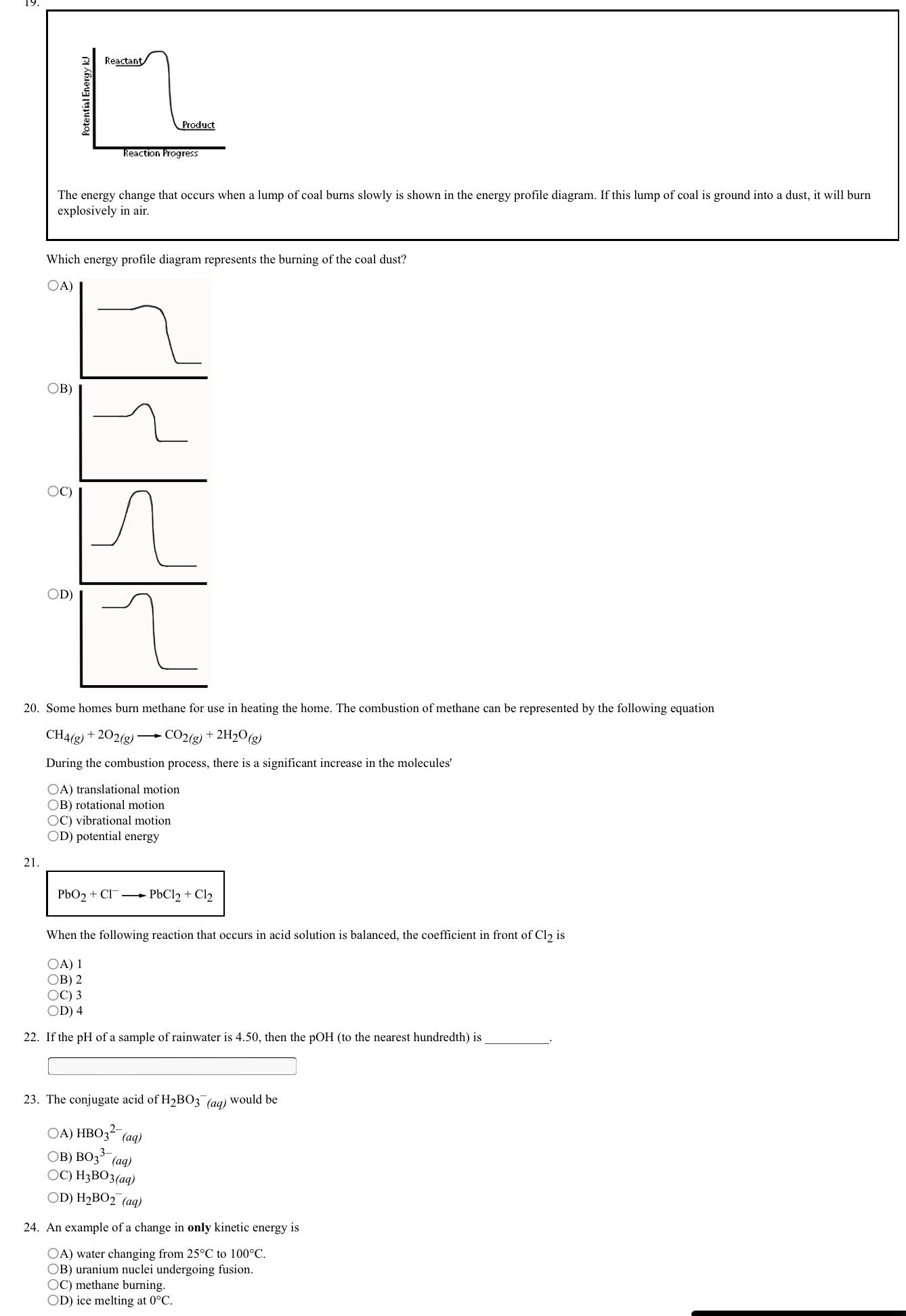
14. The reactions below involve hypothetical metals and metallic ions. Reaction Observation 2"(ag) + R(s) no evidence of reaction (aq) + T(s) evidence of reaction (aq) + S(s) evidence of reaction (aq) + S(s) no evidence of reaction [2+(ag) + Q(s) no evidence of reaction The order of the oxidizing agents, from strongest to weakest, is: OA) R2 ( ag ) , Q3+ (aq), 12+ (aq), $2 (aq) OB) T2+ (aq), Q3+ (aq), $2+ (aq), R2+ (aq) oc) Q3+ (aq), R2+ (aq), T2+ (ag), $2+ (aq) OD) R2+ (qq), $2+ (ag), Q3+ ( ag), T2+( 15. When phenol (CH5OH(s)) burns according to 2CH5OH(s) + 1402(g) - 12CO2(g) + 6H20(g) the enthalpy of reaction is -6135.8 kJ. The molar enthalpy of formation for phenol (to the nearest tenth) is kJ/mol. 16 Properties 1 Reacts spontaneously with Zn" (aq) 2 Reacts spontaneously with Ag(s) 3 Is an oxidizing and a reducing agent 4 Is reduced by Au(s) 5 Reacts spontaneously with K"(aq) 6 Reacts spontaneously with Als (ag) Which property in the list is most appropriate for Cl2(g)? 17. Salts, such as calcium chloride, which is spread onto roadways during the winter increases the rate of rust formation on vehicles because the calcium chloride OA) increases the conductivity of the electrolyte on the metal surface OB) decreases the conductivity of the electrolyte on the metal surface OC) reacts with the metal producing rust OD) reacts with the rust breaking down the metal 18. Which is a correct statement regarding any aqueous solution? OA) As [H30*(aq)] decreases, [OH (aq)] decreases OB) As [H30*(aq)] increases, [OH (aq)] increases OC) As POH increases, [OH (aq)] decreases OD) As PH increases, [H30*(aq)] increasesReactant Potential Energy KJ Product Reaction Progress The energy change that occurs when a lump of coal burns slowly is shown in the energy profile diagram. If this lump of coal is ground into a dust, it will burn explosively in air. Which energy profile diagram represents the burning of the coal dust? OA) OB) OC) OD) 20. Some homes burn methane for use in heating the home. The combustion of methane can be represented by the following equation CH4(g) + 202(g) - CO2(g) + 2H20(g) During the combustion process, there is a significant increase in the molecules' OA) translational motion OB) rotational motion OC) vibrational motion OD) potential energy 21. PbO2 + CI- - PbCl2 + Cl2 When the following reaction that occurs in acid solution is balanced, the coefficient in front of Cl2 is OA) 1 OB) 2 OC) 3 OD) 4 22. If the pH of a sample of rainwater is 4.50, then the poH (to the nearest hundredth) is 23. The conjugate acid of H2BO3 (aq) would be OA) HBO3 (aq) OB) BO3' (aq) OC) H3BO3(aq) OD) H2BO2 (aq) 24. An example of a change in only kinetic energy is OA) water changing from 25 C to 100 C. OB) uranium nuclei undergoing fusion. OC) methane burning OD) ice melting at 0 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


