Question: Can you explain please? Thank you (2 points) An acidic water sample initially contains 2mg/L of iron(III). How much iron precipitates out f the pH
Can you explain please? Thank you 
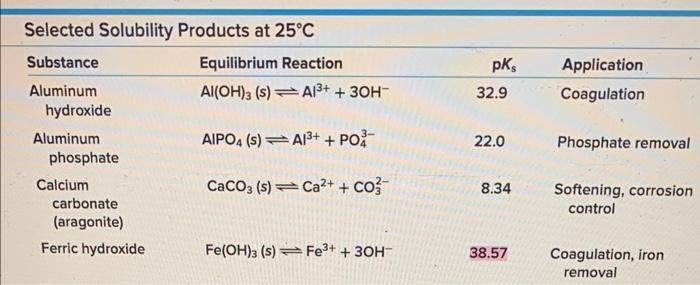


(2 points) An acidic water sample initially contains 2mg/L of iron(III). How much iron precipitates out f the pH is raised to 3 ? Assume the temperature is 25C. Refer to Table 2-1. Selected Solubility Products at 25C \begin{tabular}{llcl} Substance & Equilibrium Reaction & \multicolumn{1}{c}{pKs} & Application \\ \hline Aluminumhydroxide & Al(OH)3(s)Al3++3OH & 32.9 & Coagulation \\ Aluminumphosphate & AlPO4(s)Al3++PO43 & 22.0 & Phosphate removal \\ Calciumcarbonate(aragonite) & CaCO3(s)Ca2++CO32 & 8.34 & Softening,corrosioncontrol \\ Ferrichydroxide & Fe(OH)3(s)Fe3++3OH & 38.57 & Coagulation,ironremoval \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


