Question: can you explain why b is the correct one? i dont quiet get it Sulfamic acid, NH2SO3H, is used as a reagent in this experiment.
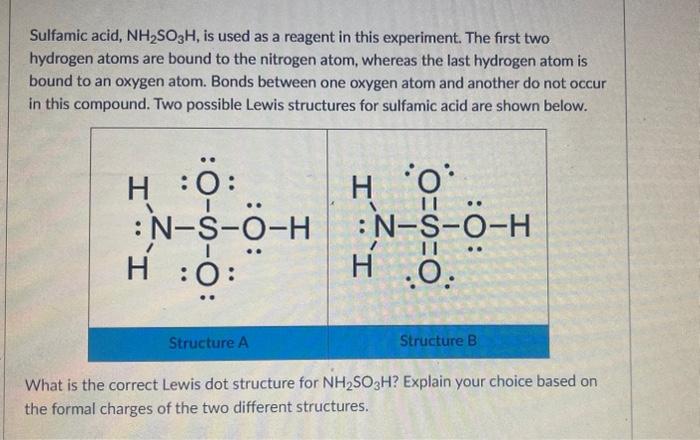
Sulfamic acid, NH2SO3H, is used as a reagent in this experiment. The first two hydrogen atoms are bound to the nitrogen atom, whereas the last hydrogen atom is bound to an oxygen atom. Bonds between one oxygen atom and another do not occur in this compound. Two possible Lewis structures for sulfamic acid are shown below. What is the correct Lewis dot structure for NH2SO3H ? Explain your choice based on the formal charges of the two different structures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


