Question: can you help me answer questions 1-3 - Experimental value - Accepted value % error = X 100% Accepted value This is a measure of
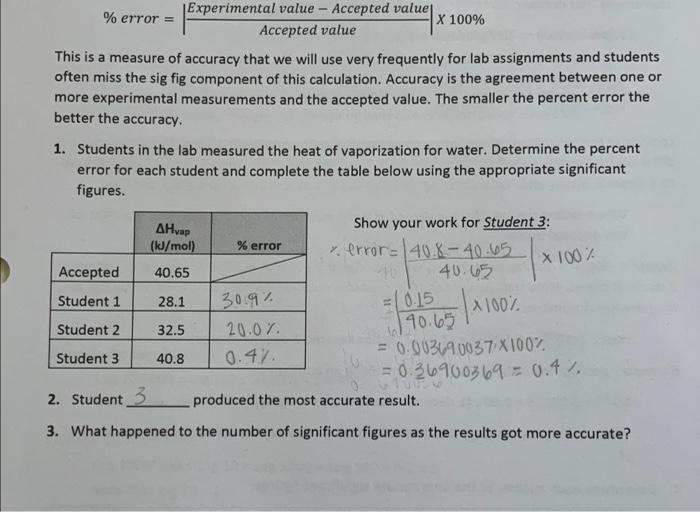
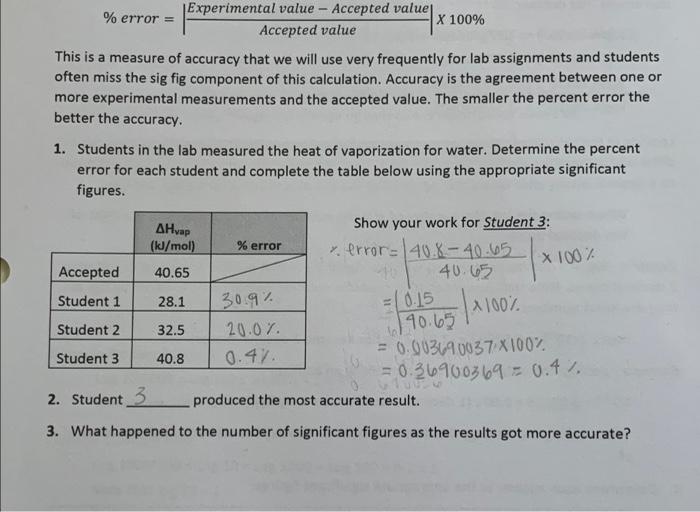
- Experimental value - Accepted value % error = X 100% Accepted value This is a measure of accuracy that we will use very frequently for lab assignments and students often miss the sig fig component of this calculation. Accuracy is the agreement between one or more experimental measurements and the accepted value. The smaller the percent error the better the accuracy. 1. Students in the lab measured the heat of vaporization for water. Determine the percent error for each student and complete the table below using the appropriate significant figures. x 100% AHvap Show your work for Student 3: (kJ/mal) % error v. error= 40.8-40.65 Accepted 40.65 40.65 Student 1 28.1 30.9% = 0.15 Student 2 32.5 20.01. 190.65 Student 3 = 0.003690037X100% 40.8 0.47 = 0.36900369 = 0.4% 2. Student 3 produced the most accurate result. 4100% 3. What happened to the number of significant figures as the results got more accurate? - Experimental value - Accepted value % error = X 100% Accepted value This is a measure of accuracy that we will use very frequently for lab assignments and students often miss the sig fig component of this calculation. Accuracy is the agreement between one or more experimental measurements and the accepted value. The smaller the percent error the better the accuracy. 1. Students in the lab measured the heat of vaporization for water. Determine the percent error for each student and complete the table below using the appropriate significant figures. x 100% AHvap Show your work for Student 3: (kJ/mal) % error v. error= 40.8-40.65 Accepted 40.65 40.65 Student 1 28.1 30.9% = 0.15 Student 2 32.5 20.01. 190.65 Student 3 = 0.003690037X100% 40.8 0.47 = 0.36900369 = 0.4% 2. Student 3 produced the most accurate result. 4100% 3. What happened to the number of significant figures as the results got more accurate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


