Question: Hello tutors! Please help me with this. This experiment is all about Freezing and Boiling Point What I need: Answers to the table Answers to





Hello tutors! Please help me with this.
This experiment is all about Freezing and Boiling Point

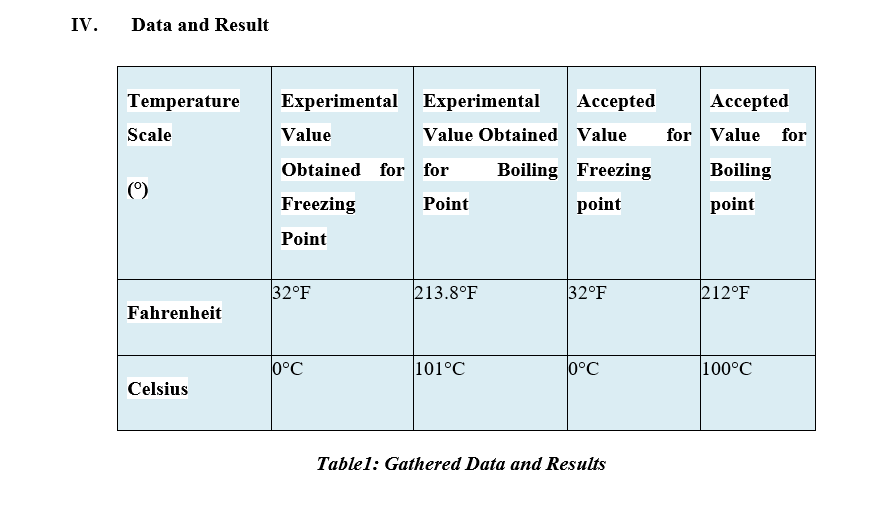





What I need: Answers to the table Answers to the 4 questions Analysis Conclusion IV. Data and Result Temperature Experimental Experimental Accepted Accepted Scale Value Value Obtained Value for Value for Obtained for for Boiling Freezing Boiling (0) Freezing Point point point Point 32.F 213.8 F 32 F 212 F Fahrenheit 101 C 100 C Celsius Table1: Gathered Data and ResultsCalculations: a) Boiling Point: b) Freezing Point: Celsius to Fahrenheit = = (C) + 32 Celsius to Fahrenheit: = (C) + 32 Percent of Error = Experimental Value - Accepted Value x 100 Accepted Value Experimental Value - Accepted Value -X 100 Experimental Value - Accepted Value -X 100 Accepted Value Accepted Value a) FREEZING POINT: (in Degree Celsius) a) BOILING POINT: (in Degree Celsius) : (in Fahrenheit) : (in Fahrenheit) VI. Answers to Questions 1. What makes thermal energy different from heat? ANSWER:D ate Performed: I. II. III. A. l . Experiment 1 Freezing and Boiling Point Objectives To test the freezing and boiling point of a thermometer. App aratusfMate-rials Iron ring Iron stand Ice Medium-sized funnel Thermometer lmlmmmh LIquld-in-ulass Thermometer B arom eter Round ask Distilled water Hot plate Gas Thumometer ' Remnant-:5 Thermometer Procedure Freezing Point Insert the thermometer into a funnel containing clean ice broken into small pieces, packing the ice well about it. 2. Allow it to remain for some minutes, until the mercury or alcohol will fall no further, and then carefully take the reading. In doing so - have the eye in such a position that a line drawn from it to top of the mercury column is at the right angles to the thermometer, and estimate the reading to tenths ofa degree. Is the thermometer graduated correctly? B. Boiling point 1. Fill the ask full of distilled water. Insert a thermometer into it. See to it that the bulb of the thermometer is approximately [cm above the water. 2. Heat the water until it boils and keep it boiling quietly so that the stem of the thermometer is surrounded by steam, which escapes through the tube. 3. Read the thermometer until the mercury or alcohol becomes steady and record its reading. Read the barometer and compute the true boiling point, being given that at 76cm, the boiling point is at 100C, and an increase of 1cm, raises the boiling point by 04C and at 29.92 inches, the boiling point is 21201:, and an increase of 1 inch raises it by 1.7013. How much error is the thermometer? Compute its % Error. IV. Data and Result Temperature Experimental Experimental Accepted Accepted Scale Value Value Obtained Value for Value for Obtained for for Boiling Freezing Boiling () Freezing Point point point Point Fahrenheil T ablel: Gathered Dam and Results 2. How do heat and temperature differ? ANSWER: 3. What are the \"fixed points" in defining a temperature scale? ANSWER: 4. What single fixed point is now used for the definition of temperature scales? ANSWER: VII. Analysis of Data VIII. Conclusion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


