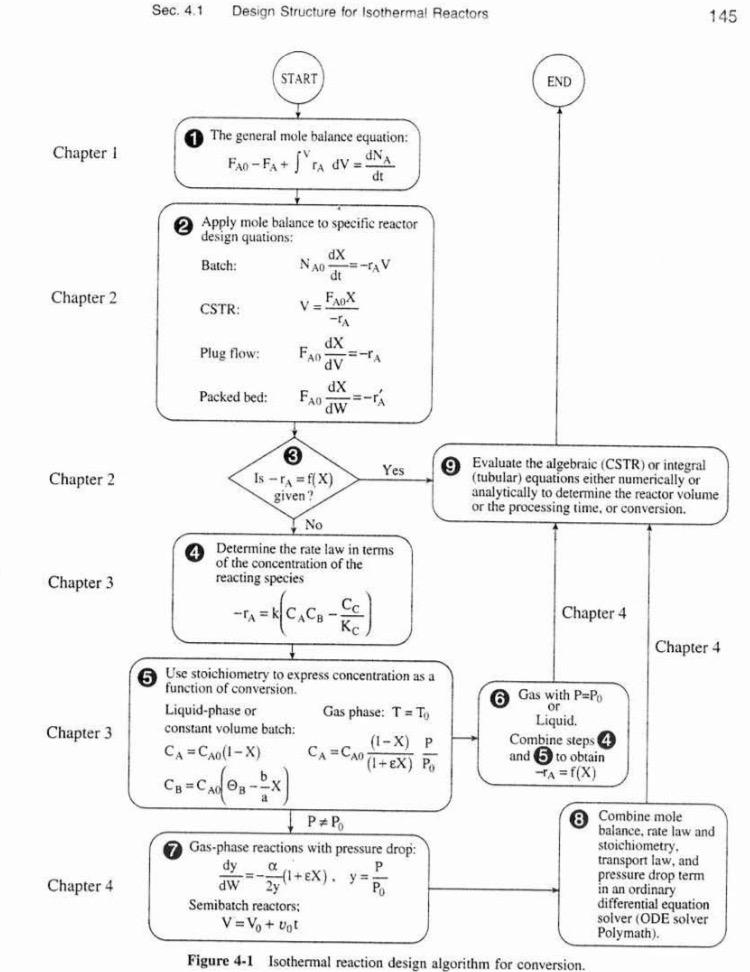
Question: can you help me just proposing the following problem P 3 - 1 7 ( Fogler 4 ta ed ) but following the steps of
can you help me just proposing the following problem PFogler ta ed but following the steps of example Fogler ta ed i put the image below to calculate how long it takes to achieve the desired conversion. THANK YOU
P B Consider a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring Figure P The reaction: A B C
with the rate expression
is taking place in this type of reactor.
a Write the rate law solely as a function of conversion, numerically evaluating all possible symbols. Ans.: lbmo
b What is the conversion and rate of reaction when Ans: lbmo
Additional information:
Equal moles of A and are present at
Initial volume:
Value of :bmol
The relationship between the volume of the reactor and pressure within the reactor is
atm
Temperature of system considered constant:
Gas constant: bmol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


