Question: can you help me pls Question 1 (1.5): A can of 5.0L of antifreeze contains 375g of water and 100g of ethylene glycol (CH2(OH)CH2(OH) ).
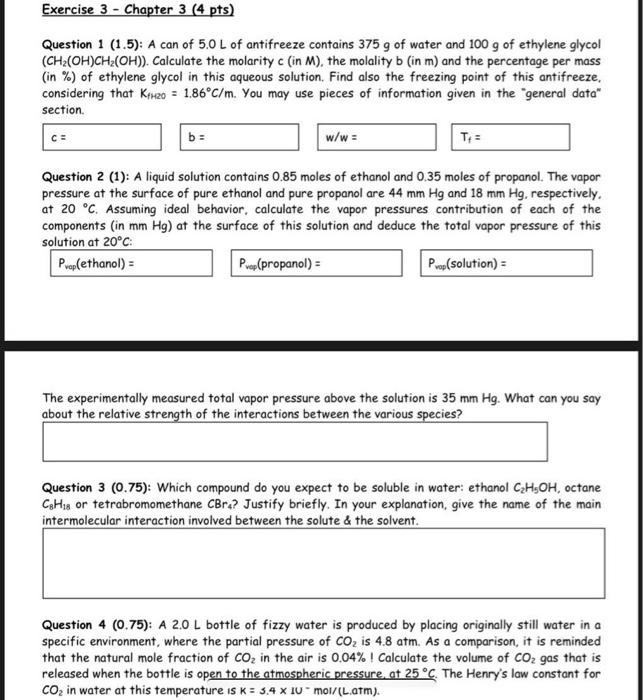
Question 1 (1.5): A can of 5.0L of antifreeze contains 375g of water and 100g of ethylene glycol (CH2(OH)CH2(OH) ). Calculate the molarity c (in M), the molality b (in m ) and the percentage per mass (in \%) of ethylene glycol in this aqueous solution. Find also the freezing point of this antifreeze, considering that KfHzO=1.86C/m. You may use pieces of information given in the "general data" section. Question 2 (1): A liquid solution contains 0.85 moles of ethanol and 0.35 moles of propanol. The vapor pressure at the surface of pure ethanol and pure propanol are 44mmHg and 18mmHg, respectively, at 20C. Assuming ideal behavior, calculate the vapor pressures contribution of each of the components (in mmHg ) at the surface of this solution and deduce the total vapor pressure of this solution at 20C The experimentally measured total vapor pressure above the solution is 35mmHg. What can you say about the relative strength of the interactions between the various species? Question 3(0.75) : Which compound do you expect to be soluble in water: ethanol C2H5OH, octane C8H18 or tetrabromomethane CBr4 ? Justify briefly. In your explanation, give the name of the main intermolecular interaction involved between the solute \& the solvent. Question 4(0.75) : A 2.0L bottle of fizzy water is produced by placing originally still water in a specific environment, where the partial pressure of CO2 is 4.8atm. As a comparison, it is reminded that the natural mole fraction of CO2 in the air is 0.04% I Calculate the volume of CO2 gas that is released when the bottle is open to the atmospheric pressure, at 25C. The Henry's law constant for CO2 in water at this temperature is K=3.41Umol/ (L.atm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


