Question: can you help me pls The air is a mixture made essentially of N2 and O2. Then calculate the air density (expressed in g/L )

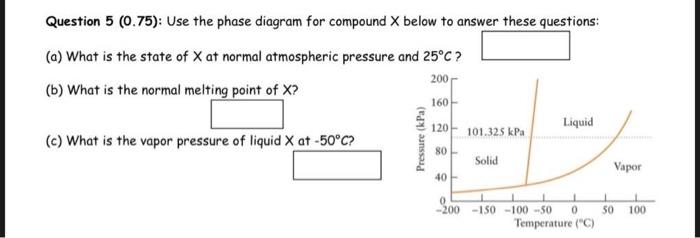
The air is a mixture made essentially of N2 and O2. Then calculate the air density (expressed in g/L ) at room pressure (P=1atm) and room temperature (T=25C). Data: mole fractions: X(N2)=80%,X(O2)=20% : molar masses: M(N2)=28g/mol,M(O2)=32g/mol. Question 2(0,5) : What is the pressure of the enclosed gas in the situation sketched on the side? The atmospheric pressure during the experiment is 1.05atm and the liquid mercury used in the syphon to measure the pressure undergoes a difference of level h=440mm. Give the answer in atm. Question 3 (1): The lead Pb(M=207.2g/mol) is a metal that crystallizes in a face-centered cubic lattice. Its density is 11.34g/cm3. Find the unit cell edge length a, expressed in A(1A=10cm). Before giving this answer, write down two important intermediate results: the number N1 of atoms per fcc cell and the number N2 of atoms per cm3 : Question 4 (1.25): A mixture of 0.05 moles of propane C3H3(g) and 0.15 moles of oxygen O2(g) is placed in a container at 27C, sealed by a piston that can move freely. The volume of the container varies such as the total pressure inside the container is always equal to 1 atm. Give the values of the partial pressures for both gases: Calculate the volume of the container in these conditions (first line of the table below). Consider now that there is an irreversible combustion reaction between propane and oxygen, producing H2O(g) and CO2(g) at 127C. Fill the advancement table above and calculate the new volume. Question 5(0.75) : Use the phase diagram for compound X below to answer these questions: (a) What is the state of X at normal atmospheric pressure and 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


