Question: Can you help solve this chart? The hydronium ion concentration of lemon juice is 3.98 . 10 moles per liter. Which statements about lemon juice
Can you help solve this chart?
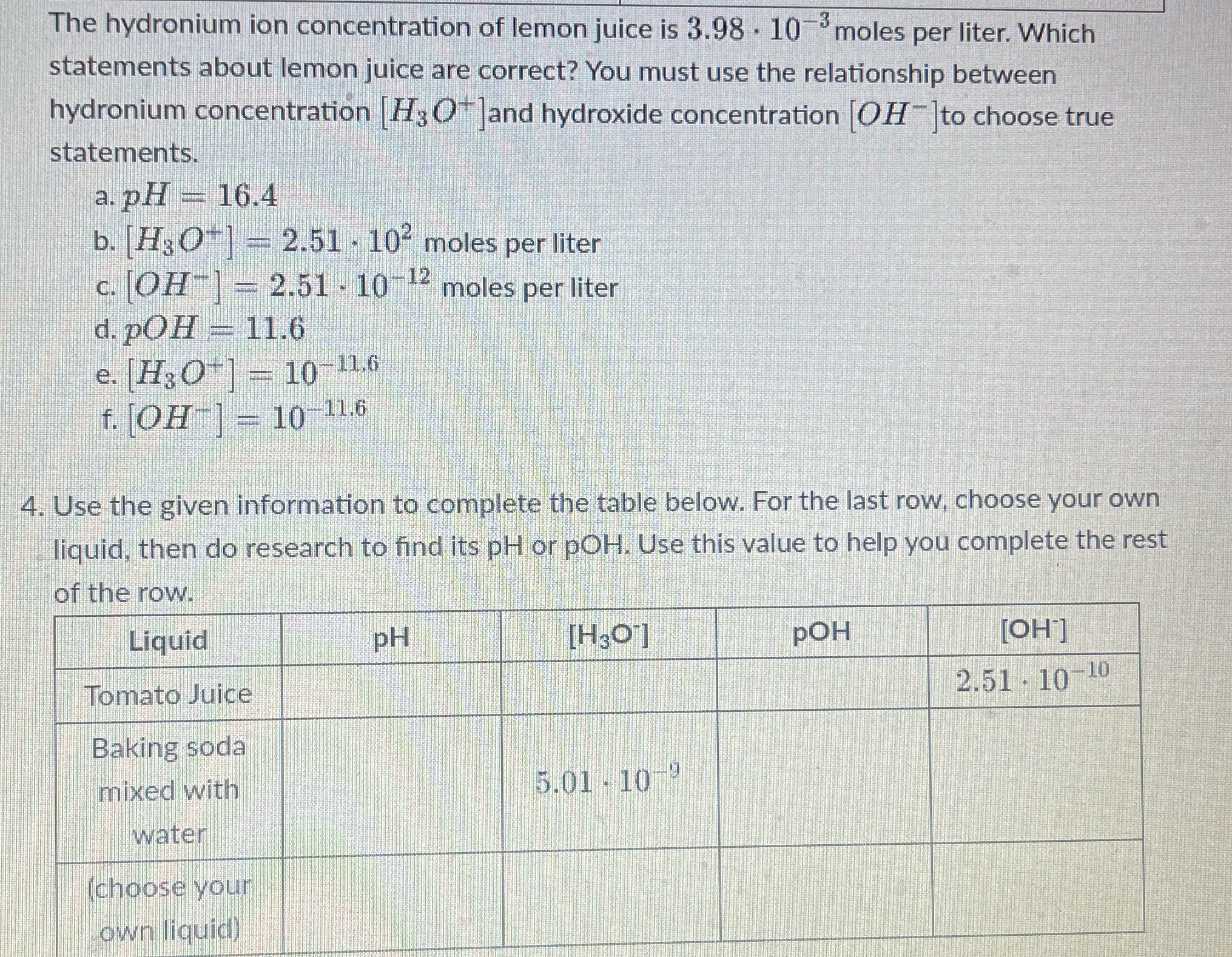
The hydronium ion concentration of lemon juice is 3.98 . 10 moles per liter. Which statements about lemon juice are correct? You must use the relationship between hydronium concentration H; O ]and hydroxide concentration [OH-]to choose true statements. a. pH - 16.4 b. Ha O*] - 2.51 - 10" moles per liter c. OH - 2.51 - 10 - moles per liter d. pOH - 11.6 e. [HO]= 10-11.6 f. OH = 10 11.6 4. Use the given information to complete the table below. For the last row, choose your own liquid, then do research to find its pH or pOH. Use this value to help you complete the rest of the row. Liquid PH [H307] POH [OH] Tomato Juice 2.51 - 10-10 Baking soda mixed with 5.01 - 10 water choose your own liquid)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


