Question: Can you help solve this ? Problem 1: Determine if the following are REDOX reaction (yeso). If so, identify which element is oxidized and which
Can you help solve this ?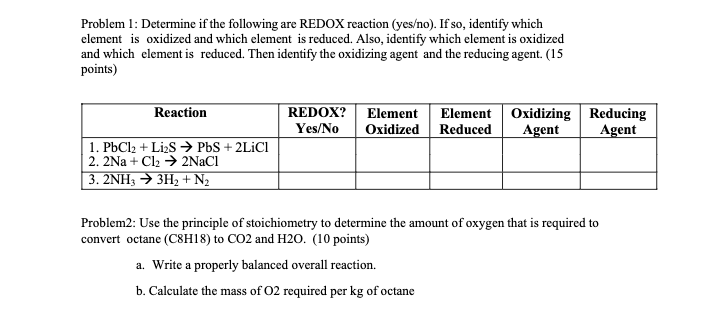
Problem 1: Determine if the following are REDOX reaction (yeso). If so, identify which element is oxidized and which element is reduced. Also, identify which element is oxidized and which element is reduced. Then identify the oxidizing agent and the reducing agent. (15 points) Problem2: Use the principle of stoichiometry to determine the amount of oxygen that is required to convert octane (C8H18) to CO2 and H2O. (10 points) a. Write a properly balanced overall reaction. b. Calculate the mass of O2 required per kg of octane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


