Question: can you help with c & d ? 5. The shell of marine organisms contain CaCO3, largely in the crystalline form known as calcite. There
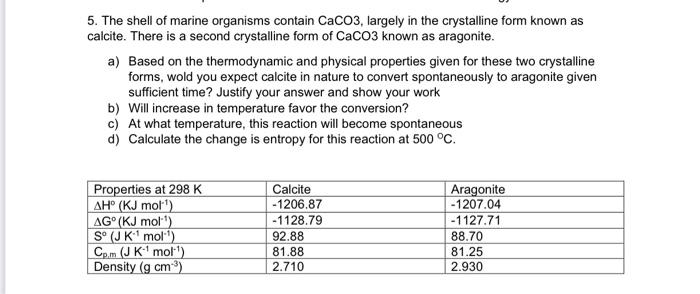
5. The shell of marine organisms contain CaCO3, largely in the crystalline form known as calcite. There is a second crystalline form of CaCO3 known as aragonite. a) Based on the thermodynamic and physical properties given for these two crystalline forms, wold you expect calcite in nature to convert spontaneously to aragonite given sufficient time? Justify your answer and show your work b) Will increase in temperature favor the conversion? c) At what temperature, this reaction will become spontaneous d) Calculate the change is entropy for this reaction at 500 C. Properties at 298 K AH (KJ molt) AG (KJ mol) SJK mol) Cp.m (JK' molt) Density (g cm) Calcite - 1206.87 -1128.79 92.88 81.88 2.710 Aragonite -1207.04 -1127.71 88.70 81.25 2.930
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


