Question: can you please help me answer i cannot figure these out The equilibrium constant, K, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) An
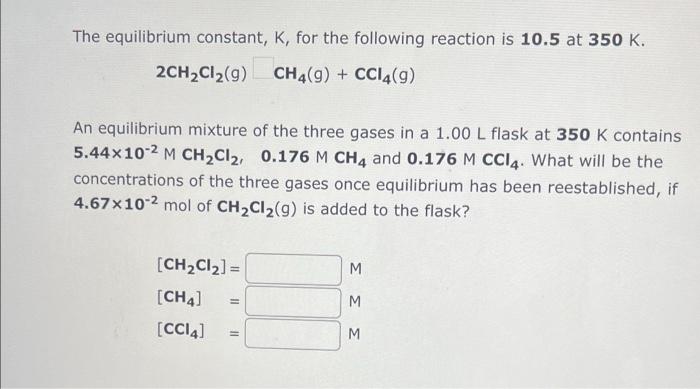
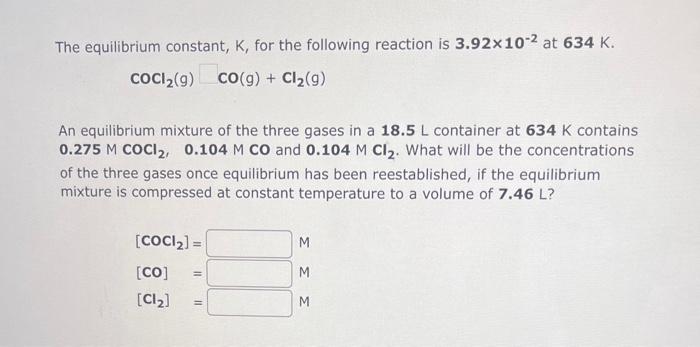
The equilibrium constant, K, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) An equilibrium mixture of the three gases in a 1.00L flask at 350K contains 5.44102MCH2Cl2,0.176MCH4 and 0.176MCCl4. What will be the concentrations of the three gases once equilibrium has been reestablished, if 4.67 102mol of CH2Cl2(g) is added to the flask? The equilibrium constant, K, for the following reaction is 3.92102 at 634K. COCl2(g)CO(g)+Cl2(g) An equilibrium mixture of the three gases in a 18.5L container at 634K contains 0.275MCOCl2,0.104MCO and 0.104MCl2. What will be the concentrations of the three gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 7.46L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


