Question: i need help for the three questions Use the References to access important values if needed for this question. Consider the following reaction: COCl2(g)CO(g)+Cl2(g) If
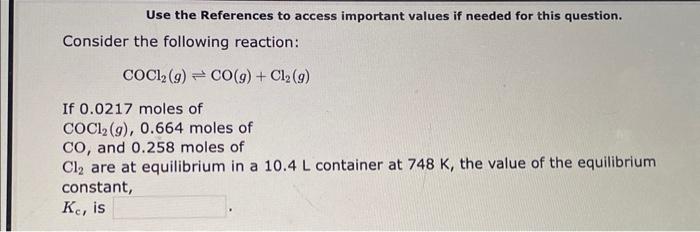


Use the References to access important values if needed for this question. Consider the following reaction: COCl2(g)CO(g)+Cl2(g) If 0.0217 moles of COCl2(g),0.664 moles of CO, and 0.258 moles of Cl2 are at equilibrium in a 10.4L container at 748K, the value of the equilibrium constant, Kc, is The equilibrium constant, Kc, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) If an equilibrium mixture of the three gases in a 17.6L container at 350K contains 0.419 mol of CH2Cl2(g) and 0.453mol of CH4, the equilibrium concentration of CCl4 is M. The equilibrium constant, Kc, for the following reaction is 0.0180 at 698K. 2HI(g)H2(g)+I2(g) If an equilibrium mixture of the three gases in a 19.5L container at 698K contains 0.298mol of HI(g) and 0.399mol of H2, the equilibrium concentration of I2 is M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


