Question: Can you please help me with this : For the HDA Process 1. Calculate the amount of heat required in E-101 to preheat the feed
Can you please help me with this :
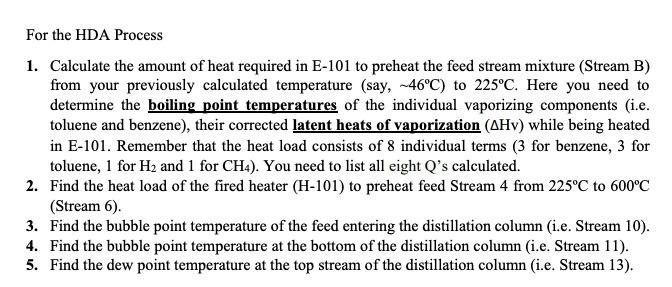
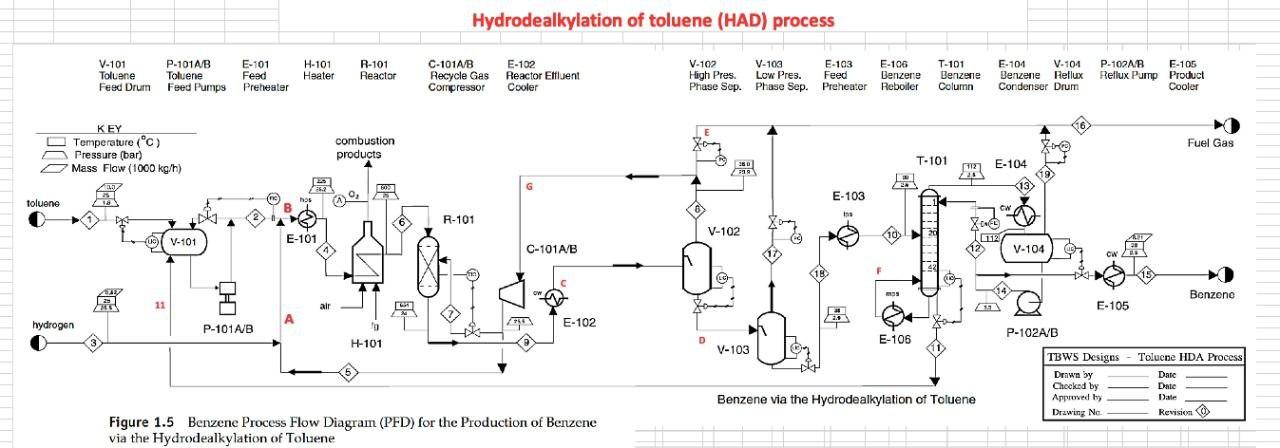
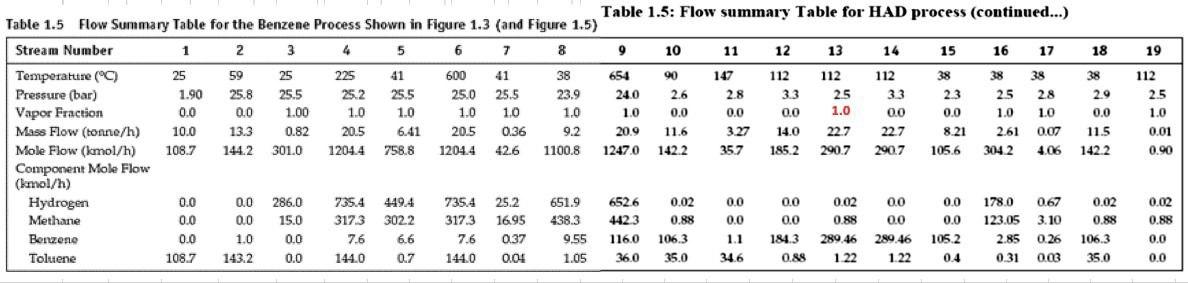
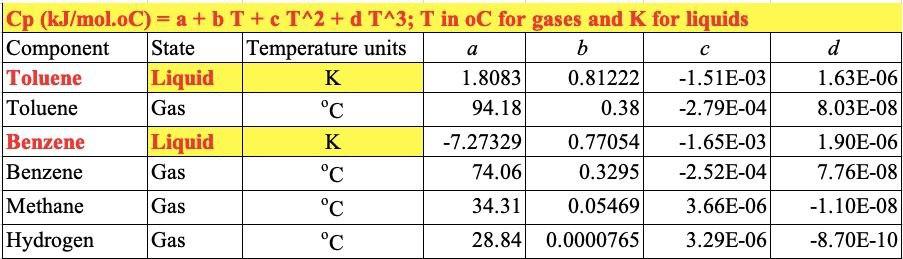
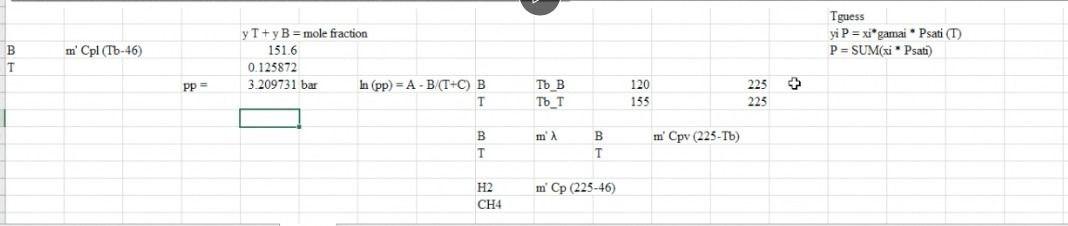
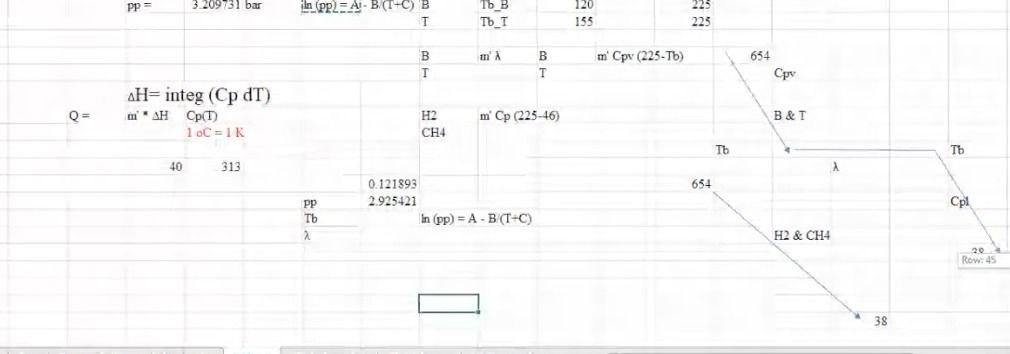
For the HDA Process 1. Calculate the amount of heat required in E-101 to preheat the feed stream mixture (Stream B) from your previously calculated temperature (say, -46C) to 225C. Here you need to determine the boiling point temperatures of the individual vaporizing components (i.e. toluene and benzene), their corrected latent heats of vaporization (AHY) while being heated in E-101. Remember that the heat load consists of 8 individual terms (3 for benzene, 3 for toluene, 1 for H2 and 1 for CH4). You need to list all eight Q's calculated. 2. Find the heat load of the fired heater (H-101) to preheat feed Stream 4 from 225C to 600C (Stream 6). 3. Find the bubble point temperature of the feed entering the distillation column (i.e. Stream 10). 4. Find the bubble point temperature at the bottom of the distillation column (i.e. Stream 11). 5. Find the dew point temperature at the top stream of the distillation column (i.e. Stream 13). Hydrodealkylation of toluene (HAD) process V-101 P-101A/B Toluene Toluene Feed Drum Feed Pumps E-101 Feed Preheater H-101 Heater R-101 Reactor C-101A/B Recycle Gas Compressor E-102 Reactor Effluent Cooler V-102 V-103 E-103 F-106 High Pres. Low Pres. Feed Benzerte Phase Sup. Phase Sep. Preheater Reboiler T-101 Benzene Column E-104 V-101 Benzene Reflux Condenser Drum P-102A/B F-105 Reflux Pump Product Cooler Fuel Gas KEY O Temperature (C) Pressure (bar) Mass Flow (1000 kg/h) combustion products T-101 E-104 529 E-103 toluene hos R-101 V-102 Xood I.12 V-101 uc) E-101 E 10 C-101A/B 17 V-104 19 F Benzone 11 air E-105 hydrogen E-102 P-101A/B 19 H-101 P-102A/B D E-106 V-103 TBWS Designs Drawn by Checked by Approved by Drawing No Toluene HDA Process Datc Date Date Revision Benzene via the Hydrode alkylation of Toluene Figure 1.5 Benzene Process Flow Diagram (PFD) for the Production of Benzene via the Hydrodealkylation of Toluene 19 112 112 112 25 1.0 0.01 Table 1.5: Flow summary Table for HAD process (continued...) Table 1.5 Flow Summary Table for the Benzene Process Shown in Figure 1.3 (and Figure 1.5) Stream Number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Temperature (C) 25 59 25 225 41 600 41 38 654 90 147 112 38 38 38 38 Pressure (bar) 1.90 25.8 25.5 25.2 25.5 25.0 25.5 23.9 24.0 26 2.8 3.3 25 3.3 23 25 28 2.9 Vapor Fraction 0.0 0.0 1.00 1.0 1.0 1.0 1.0 1.0 1.0 0.0 0.0 0.0 1.0 0.0 0.0 1.0 1.0 0.0 Mass Flow (tonne/h) 10.0 0.82 20.5 6.41 0.36 9.2 20.9 11.6 3.27 14.0 22.7 22.7 8.21 2.61 0.07 11.5 Mole Flow (kmol/h) 108.7 144.2 3010 1204.4 758.8 12044 426 1100.8 12470 142.2 357 185.2 290.7 290.7 105.6 304.2 4.06 142.2 Component Mole Flow (kmol/h) Hydrogen 0.0 0.0 286.0 735.4 449.4 735.4 25.2 651.9 652.6 0.02 0.0 0.0 0.02 0.0 0.0 178.0 0.67 0.02 Methane 0.0 0.0 15.0 3173 3022 3173 1695 438.3 442.3 0.88 0.0 0.0 0.88 0.0 0.0 123.05 3.10 0.88 Benzene 0.0 1.0 0.0 7.6 6.6 7.6 0.37 9.55 116.0 106,3 1.1 1843 289.46 289.46 1052 2.85 0.26 106.3 Toluene 108.7 143.2 0.0 144.0 0.7 144.0 0.04 1.05 360 35.0 346 0.88 1.22 1.22 0.4 0.31 0.03 35.0 13.3 20.5 0.90 0.02 0.88 0.0 0.0 d 1.63E-06 8.03E-08 Cp (kJ/mol.oC)= a + b T+ c T^2+ d T^3; T in oC for gases and K for liquids Component State Temperature units a b Toluene Liquid K 1.8083 0.81222 -1.51E-03 Toluene Gas C 94.18 0.38 -2.79E-04 Benzene Liquid K -7.27329 0.77054 -1.65E-03 Benzene Gas C 74.06 0.3295 -2.52E-04 Methane Gas C 34.31 0.05469 3.66E-06 Hydrogen Gas C 28.84 0.0000765 3.29E-06 1.90E-06 7.76E-08 -1.10E-08 -8.70E-10 Tguess yi P = xi gamai Psati (1) P = SUM(xi Psati m' Cpl (Tb-46) B T y T+ y B = mole fraction 151.6 0.125872 3.209731 bar In (pp) = A - B/(T+C) B T + Tb B To_T 120 135 225 225 m B B T m' Cpv (225-Tb) H2 CH4 m' Cp (225-46) PP = 320931 bar in pp2=AI-BTC) 1 155 223 in B T mi" (225-) 654 Q= = integ (p dT) ) 1 oC = 1K ? CH4 m" (225-46) B&T T T 40 313 654 0.121893 2.925421 h (pp) = A - BT-C) PP pl H & CHI 20 Row: 45
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


