Question: can you please help with with 1 and 2 on a separate sheet of paper Please review the background knowledge posted on iCollege. Answer the
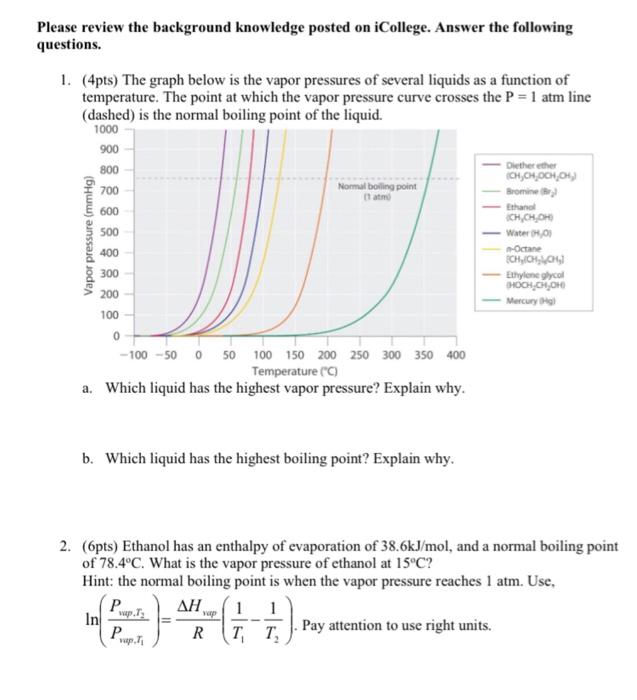
Please review the background knowledge posted on iCollege. Answer the following questions. 1. (4pts) The graph below is the vapor pressures of several liquids as a function of temperature. The point at which the vapor pressure curve crosses the P=1atm line (dashed) is the normal boiling point of the liquid. b. Which liquid has the highest boiling point? Explain why. 2. (6pts) Ethanol has an enthalpy of evaporation of 38.6kJ/mol, and a normal boiling point of 78.4C. What is the vapor pressure of ethanol at 15C ? Hint: the normal boiling point is when the vapor pressure reaches 1atm. Use, ln(Pvap,T1Pvap,T2)=RHvap(T11T21). Pay attention to use right units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


