Question: can you please solve these problems with steps PART 1. DETERMINATION OF THE HEAT CAPACITY OF THE CALORIMETER EXPERIMENTAL DATA - The specilic heal edpacity
can you please solve these problems with steps
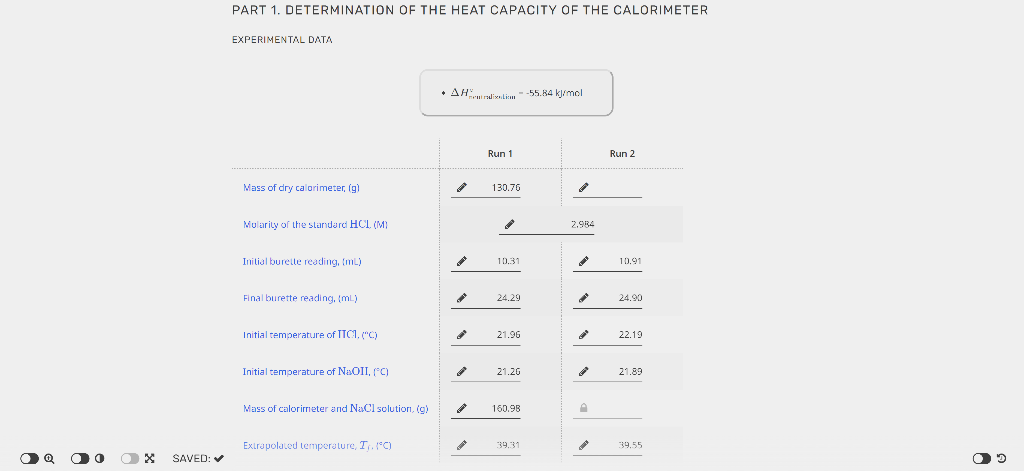
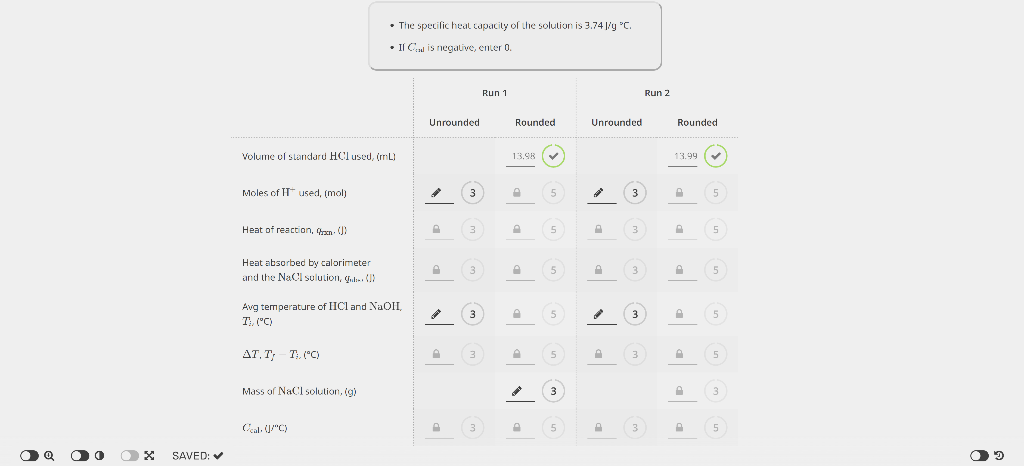
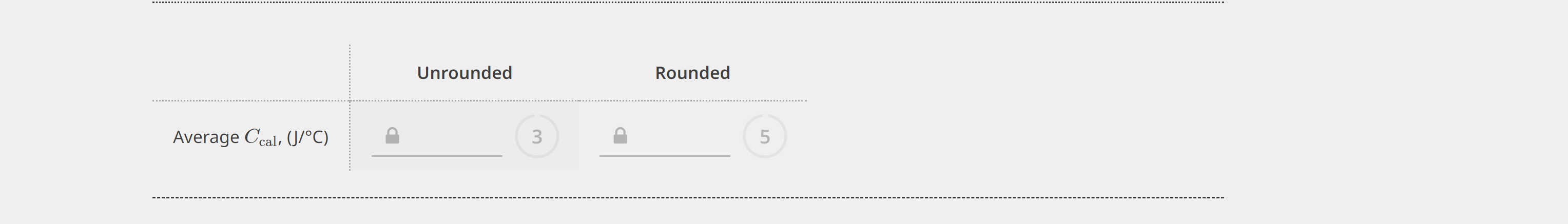
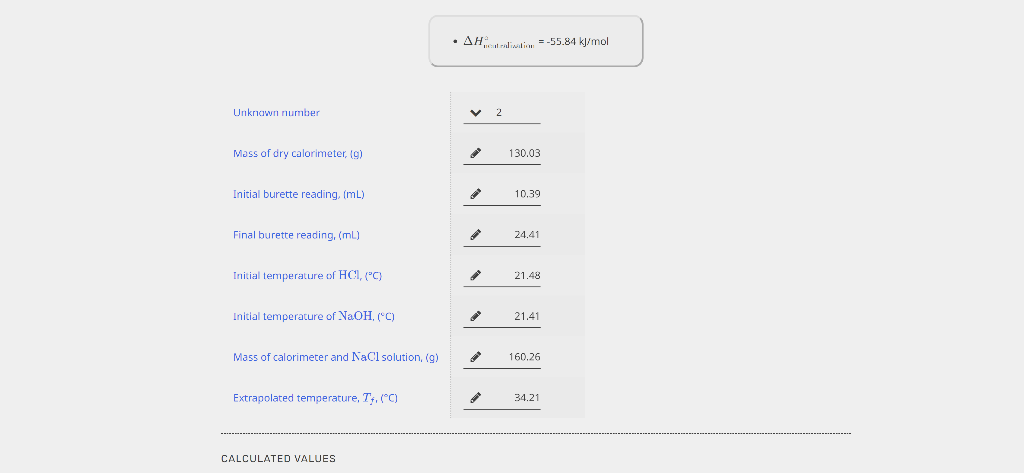
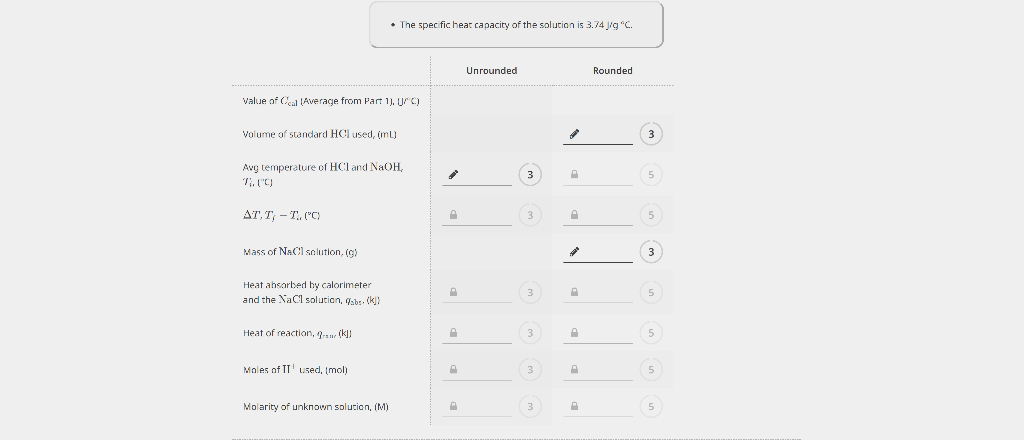
PART 1. DETERMINATION OF THE HEAT CAPACITY OF THE CALORIMETER EXPERIMENTAL DATA - The specilic heal edpacity of the solution is 3.74J/yC. - Ir Ctal is negalve, enter 0. Unrounded Rounded Average Ccal,(J/C) 3 5 Unknown number Mass of dry calorimeter, (g) Initial burette reading, (mL) Final burette reading, (mL) Initial temperature of HCl,{C} Initial temperacure of NaOH,(C) Mass of calorimeter and NaCl solution, (g) Extrapolated temperature, Tf,C ] 10.3924.41 21.48 21,41 CALCULATED VALUES - The specicic heac capacity of the solucion is 3.74JgC. Value of Ccal (Average from Part 1), Uf C ) Volume of standard HCl used imLI2 Avg temperature of HCl and NaOHr Heat ahsorbed by calorimeter and the XaCl solution, qsbs,[k]} (3) Heat of reaction, qumik]l Males af II' used, (mol) Molarity of unknown solution, (M) PART 1. DETERMINATION OF THE HEAT CAPACITY OF THE CALORIMETER EXPERIMENTAL DATA - The specilic heal edpacity of the solution is 3.74J/yC. - Ir Ctal is negalve, enter 0. Unrounded Rounded Average Ccal,(J/C) 3 5 Unknown number Mass of dry calorimeter, (g) Initial burette reading, (mL) Final burette reading, (mL) Initial temperature of HCl,{C} Initial temperacure of NaOH,(C) Mass of calorimeter and NaCl solution, (g) Extrapolated temperature, Tf,C ] 10.3924.41 21.48 21,41 CALCULATED VALUES - The specicic heac capacity of the solucion is 3.74JgC. Value of Ccal (Average from Part 1), Uf C ) Volume of standard HCl used imLI2 Avg temperature of HCl and NaOHr Heat ahsorbed by calorimeter and the XaCl solution, qsbs,[k]} (3) Heat of reaction, qumik]l Males af II' used, (mol) Molarity of unknown solution, (M)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


