Question: can you please use CHEMCAD 7 software to simulate and answer the question (20,000 kg/h) from 200C (at its bubble point pressure) to 93C using

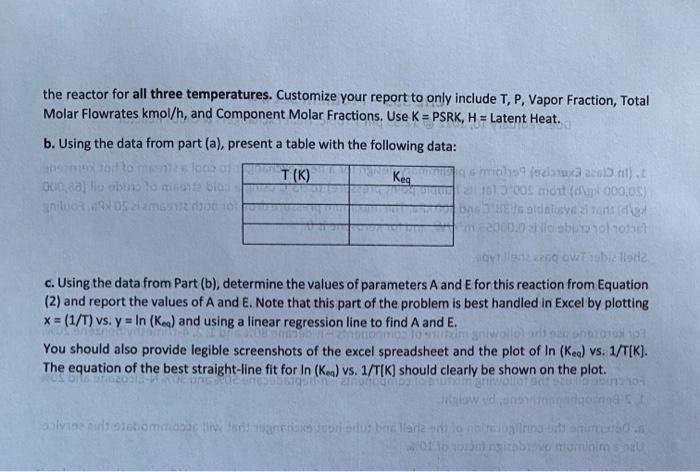
(20,000 kg/h) from 200C (at its bubble point pressure) to 93C using a cold stream of crude oil (68.000 1. (In Class Exercise) Perform a preliminary design for a heat exchanger to cool a stream of hot kerosene kg/h) that is available at 38C and 500 kPa. The maximum pressure drop for both streams is 20 kPa. Fouling factor for crude oil is 0.00053 mK/W, and for kerosene is 0. Shell side: Two pass shell type Tube side: 1-inch outer diameter, 14 ft long (fixed), 12 BWG, carbon steel tubes. Tube pitch 1.25 inch. 1- inch tubesheet thickness. 2 tubesheets used. Crude oil flows in the tubes. For kerosene use the following mixture of components-n-decane 80% and 1,2,4-trimethylbenzene 20%. by weight For crude oil use the following mixture of components-n-heptadecane 50% and 30% N-Eicosane and 20% 1, 2-Benzophenanthrene, by weight. a. Determine the configuration of the shell and tube heat exchanger that will accommodate this service. Use a minimum overdesign factor of 10%. b. With the design found in Part (a) do a simulation of the heat exchanger. What are the actual exit temperatures and pressure drops across the exchanger? Note: Many different results are possible depending on what additional things you specify. The grading will be based on if the above given info is correctly set up in CHEMCAD. 2. The production of Dimethyl Ether via the dehydration of methanol takes place in the gas phase at temperatures in the range 250 to 400*C. 2CH, OH (CH3)20 + H20 (1) methanol OME water This is a reversible, exothermic reaction. YONEXwater The equilibrium coefficient for this reaction keq is given as Keq = A exp(12) (XMEON) where x is the molar fraction of component i and the right-hand side of Keq Equation is a function of TIK) only and A and E are to be determined. For this reaction do the following: a. Starting with a feed of 100 kmol/h of methanol simulate the reaction using a Gibbs reactor at a pressure of 15 bar and temperatures of 200C, 300C, and 400*C. For the Gibbs reactor, you can only provide the screenshot for 200C. Please show the stream compositions for the feed. Please also show the outlet from the reactor for all three temperatures. Customize your report to only include T, P, Vapor Fraction, Total Molar Flowrates kmol/h, and Component Molar Fractions. Use K = PSRK, H = Latent Heat. b. Using the data from part (a), present a table with the following data: otomeT(K). Keg masina 2011) Ooo, a lo obto lo son los 7) 3005 mot 00.05) na 2020 soldalovazione 2000.00 Obica c. Using the data from Part (b), determine the values of parameters A and E for this reaction from Equation (2) and report the values of A and E. Note that this part of the problem is best handled in Excel by plotting x = (1/T) vs. y = In (Kea) and using a linear regression line to find A and E. You should also provide legible screenshots of the excel spreadsheet and the plot of in (Kea) vs. 1/T[K). The equation of the best straight-line fit for In (Kea) vs. 1/T(K) should clearly be shown on the plot. 10- ingens Veidoscommodationer bros barle long run 0110 al sitoomine (20,000 kg/h) from 200C (at its bubble point pressure) to 93C using a cold stream of crude oil (68.000 1. (In Class Exercise) Perform a preliminary design for a heat exchanger to cool a stream of hot kerosene kg/h) that is available at 38C and 500 kPa. The maximum pressure drop for both streams is 20 kPa. Fouling factor for crude oil is 0.00053 mK/W, and for kerosene is 0. Shell side: Two pass shell type Tube side: 1-inch outer diameter, 14 ft long (fixed), 12 BWG, carbon steel tubes. Tube pitch 1.25 inch. 1- inch tubesheet thickness. 2 tubesheets used. Crude oil flows in the tubes. For kerosene use the following mixture of components-n-decane 80% and 1,2,4-trimethylbenzene 20%. by weight For crude oil use the following mixture of components-n-heptadecane 50% and 30% N-Eicosane and 20% 1, 2-Benzophenanthrene, by weight. a. Determine the configuration of the shell and tube heat exchanger that will accommodate this service. Use a minimum overdesign factor of 10%. b. With the design found in Part (a) do a simulation of the heat exchanger. What are the actual exit temperatures and pressure drops across the exchanger? Note: Many different results are possible depending on what additional things you specify. The grading will be based on if the above given info is correctly set up in CHEMCAD. 2. The production of Dimethyl Ether via the dehydration of methanol takes place in the gas phase at temperatures in the range 250 to 400*C. 2CH, OH (CH3)20 + H20 (1) methanol OME water This is a reversible, exothermic reaction. YONEXwater The equilibrium coefficient for this reaction keq is given as Keq = A exp(12) (XMEON) where x is the molar fraction of component i and the right-hand side of Keq Equation is a function of TIK) only and A and E are to be determined. For this reaction do the following: a. Starting with a feed of 100 kmol/h of methanol simulate the reaction using a Gibbs reactor at a pressure of 15 bar and temperatures of 200C, 300C, and 400*C. For the Gibbs reactor, you can only provide the screenshot for 200C. Please show the stream compositions for the feed. Please also show the outlet from the reactor for all three temperatures. Customize your report to only include T, P, Vapor Fraction, Total Molar Flowrates kmol/h, and Component Molar Fractions. Use K = PSRK, H = Latent Heat. b. Using the data from part (a), present a table with the following data: otomeT(K). Keg masina 2011) Ooo, a lo obto lo son los 7) 3005 mot 00.05) na 2020 soldalovazione 2000.00 Obica c. Using the data from Part (b), determine the values of parameters A and E for this reaction from Equation (2) and report the values of A and E. Note that this part of the problem is best handled in Excel by plotting x = (1/T) vs. y = In (Kea) and using a linear regression line to find A and E. You should also provide legible screenshots of the excel spreadsheet and the plot of in (Kea) vs. 1/T[K). The equation of the best straight-line fit for In (Kea) vs. 1/T(K) should clearly be shown on the plot. 10- ingens Veidoscommodationer bros barle long run 0110 al sitoomine
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


